当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Functional Characterization of a Condensation Domain That Links Nonribosomal Peptide and Pteridine Biosynthetic Machineries in Photorhabdus luminescens
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.biochem.7b00863 Corey E. Perez 1, 2 , Hyun Bong Park 1, 2 , Jason M. Crawford 1, 2, 3
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.biochem.7b00863 Corey E. Perez 1, 2 , Hyun Bong Park 1, 2 , Jason M. Crawford 1, 2, 3
Affiliation
|
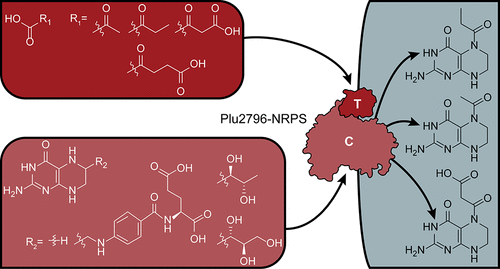
Nonribosomal peptide synthetases (NRPSs) produce a wide variety of biologically important small molecules. NRPSs can interface with other enzymes to form hybrid biosynthetic systems that expand the structural and functional diversity of their products. The pepteridines are metabolites encoded by an unprecedented pteridine–NRPS-type hybrid biosynthetic gene cluster in Photorhabdus luminescens, but how the distinct enzymatic systems interface to produce these molecules has not been examined at the biochemical level. By an unknown mechanism, the genetic locus can also affect the regulation of other enzymes involved in autoinducer and secondary metabolite biosynthesis. Here, through in vitro protein biochemical assays, we demonstrate that an atypical NRPS condensation (C) domain present in the pathway condenses acyl units derived from α-keto acids onto a free 5,6,7,8-tetrahydropterin core, producing the tertiary cis-amide-containing pepteridines. Solution studies of the chemically synthesized molecules led to the same amide regiochemistries that were observed in the natural products. The biochemical transformations mediated by the C domain destroy the radical scavenging activity of its redox active tetrahydropterin substrate. Secondary metabolite analyses revealed that the pepteridine locus affects select metabolic pathways associated with quorum sensing, antibiosis, and symbiosis. Taken together, the results suggest that the pathway likely regulates cellular redox and specialized metabolic pathways through engagement with the citric acid cycle.
中文翻译:

链接非核糖体肽和蝶啶生物合成机制在光致发光中的缩合域的功能表征。
非核糖体肽合成酶(NRPS)产生各种生物学上重要的小分子。NRPS可以与其他酶相互作用形成混合生物合成系统,从而扩展其产品的结构和功能多样性。肽苷是光致发光菌中前所未有的蝶啶-NRPS型杂化生物合成基因簇编码的代谢物,但尚未在生化水平上检验独特的酶系统如何相互作用以产生这些分子。通过未知的机制,遗传位点还可以影响涉及自动诱导剂和次级代谢产物生物合成的其他酶的调控。在这里,通过体外蛋白质生化分析,我们证明了该途径中存在的非典型NRPS缩合(C)结构域将衍生自α-酮酸的酰基单元缩合到自由的5,6,7,8-四氢蝶呤核心上,从而产生叔顺式-含酰胺的肽核苷。化学合成分子的溶液研究导致了与天然产物中观察到的相同的酰胺区域化学反应。由C结构域介导的生化转化破坏了其氧化还原活性四氢蝶呤底物的自由基清除活性。二级代谢产物分析表明,肽吡啶基因座会影响与群体感应,抗菌和共生相关的某些代谢途径。两者合计,结果表明该途径可能通过参与柠檬酸循环来调节细胞的氧化还原和专门的代谢途径。
更新日期:2018-01-16
中文翻译:

链接非核糖体肽和蝶啶生物合成机制在光致发光中的缩合域的功能表征。
非核糖体肽合成酶(NRPS)产生各种生物学上重要的小分子。NRPS可以与其他酶相互作用形成混合生物合成系统,从而扩展其产品的结构和功能多样性。肽苷是光致发光菌中前所未有的蝶啶-NRPS型杂化生物合成基因簇编码的代谢物,但尚未在生化水平上检验独特的酶系统如何相互作用以产生这些分子。通过未知的机制,遗传位点还可以影响涉及自动诱导剂和次级代谢产物生物合成的其他酶的调控。在这里,通过体外蛋白质生化分析,我们证明了该途径中存在的非典型NRPS缩合(C)结构域将衍生自α-酮酸的酰基单元缩合到自由的5,6,7,8-四氢蝶呤核心上,从而产生叔顺式-含酰胺的肽核苷。化学合成分子的溶液研究导致了与天然产物中观察到的相同的酰胺区域化学反应。由C结构域介导的生化转化破坏了其氧化还原活性四氢蝶呤底物的自由基清除活性。二级代谢产物分析表明,肽吡啶基因座会影响与群体感应,抗菌和共生相关的某些代谢途径。两者合计,结果表明该途径可能通过参与柠檬酸循环来调节细胞的氧化还原和专门的代谢途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号