当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric [3+2] Annulations to Construct 1,2-Bispirooxindoles Incorporating a Dihydropyrrolidine Motif
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-11-06 09:05:44 , DOI: 10.1002/adsc.201700849 Qing He 1 , Wei Du 1 , Ying-Chun Chen 1, 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-11-06 09:05:44 , DOI: 10.1002/adsc.201700849 Qing He 1 , Wei Du 1 , Ying-Chun Chen 1, 2
Affiliation
|
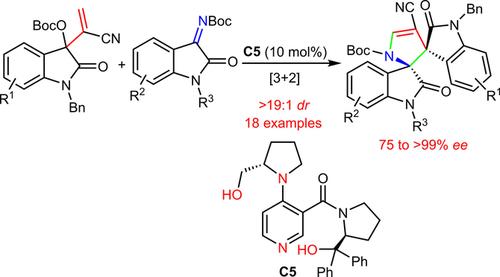
Constructing chiral bispirooxindoles is difficult to achieve but highly attractive owing to their many potential applications in medicinal chemistry. Here we present an asymmetric [3+2] annulation reaction of Morita–Baylis–Hillman carbonates from isatins and isatin-based N-Boc-ketimines under the catalysis of a newly designed multifunctional 4-dimethylaminopyridine-type substance. The reaction shows high γ-regioselectivity, producing highly complex 1,2-bispirooxindoles incorporating a dihydropyrrolidine motif in excellent yields with moderate to outstanding stereoselectivity (dr>19:1, up to >99% ee). This protocol has been expanded to utilize trifluoromethyl-containing ketimines, delivering complicated architectures with fused and spirocyclic frameworks in modest enantioselectivity.
中文翻译:

不对称的[3 + 2]环进构筑包含二氢吡咯烷基的1,2-Bispirooxindoles
构造手性双螺硫辛酯很难实现,但由于其在药物化学中的许多潜在应用,因此具有很高的吸引力。在这里,我们介绍了新设计的多功能4-二甲基氨基吡啶类物质催化下,由靛红和基于靛红的N - Boc-酮亚胺组成的Morita-Baylis-Hillman碳酸盐的不对称[3 + 2]环化反应。该反应显示出较高的γ-区域选择性,可生产出具有二氢吡咯烷基序的高度复杂的1,2-bispirooxindoles,并具有中等至出色的立体选择性(dr > 19:1,高达> 99%ee))。该协议已扩展为利用含三氟甲基的酮亚胺,以适度的对映选择性提供具有稠合和螺环骨架的复杂结构。
更新日期:2017-11-06
中文翻译:

不对称的[3 + 2]环进构筑包含二氢吡咯烷基的1,2-Bispirooxindoles
构造手性双螺硫辛酯很难实现,但由于其在药物化学中的许多潜在应用,因此具有很高的吸引力。在这里,我们介绍了新设计的多功能4-二甲基氨基吡啶类物质催化下,由靛红和基于靛红的N - Boc-酮亚胺组成的Morita-Baylis-Hillman碳酸盐的不对称[3 + 2]环化反应。该反应显示出较高的γ-区域选择性,可生产出具有二氢吡咯烷基序的高度复杂的1,2-bispirooxindoles,并具有中等至出色的立体选择性(dr > 19:1,高达> 99%ee))。该协议已扩展为利用含三氟甲基的酮亚胺,以适度的对映选择性提供具有稠合和螺环骨架的复杂结构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号