当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Amide synthesis via nickel-catalysed reductive aminocarbonylation of aryl halides with nitroarenes†
Chemical Science ( IF 7.6 ) Pub Date : 2017-11-06 00:00:00 , DOI: 10.1039/c7sc03950f Chi Wai Cheung 1 , Marten Leendert Ploeger 1 , Xile Hu 1
Chemical Science ( IF 7.6 ) Pub Date : 2017-11-06 00:00:00 , DOI: 10.1039/c7sc03950f Chi Wai Cheung 1 , Marten Leendert Ploeger 1 , Xile Hu 1
Affiliation
|
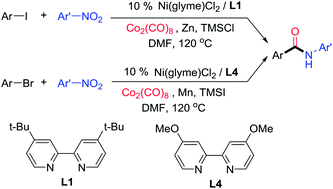
Aminocarbonylation of aryl halides is one of the most useful methods in amide synthesis, but nitroarenes have not been used as a nitrogen source in this method even though they are more economical and accessible than anilines. Reported here is the development of nickel catalysis for the first three-component reactions of aryl halides, Co2(CO)8, and nitroarenes under reductive conditions to produce aryl amides. A wide range of (hetero)aryl iodides and bromides as well as nitro(hetero)arenes are suitable reaction partners, and high functional group compatibility has been achieved. The method might be used for the streamlined synthesis of aryl amides.
中文翻译:

通过镍催化芳基卤化物与硝基芳烃的还原氨基羰基化合成酰胺†
芳基卤化物的氨基羰基化是酰胺合成中最有用的方法之一,但硝基芳烃尚未在该方法中用作氮源,尽管它们比苯胺更经济且更容易获得。本文报道了镍催化的发展,用于芳基卤化物、Co 2 (CO) 8和硝基芳烃在还原条件下生产芳基酰胺的第一个三组分反应。多种(杂)芳基碘化物和溴化物以及硝基(杂)芳烃是合适的反应伙伴,并且已经实现了高官能团相容性。该方法可用于芳基酰胺的简化合成。
更新日期:2017-11-06
中文翻译:

通过镍催化芳基卤化物与硝基芳烃的还原氨基羰基化合成酰胺†
芳基卤化物的氨基羰基化是酰胺合成中最有用的方法之一,但硝基芳烃尚未在该方法中用作氮源,尽管它们比苯胺更经济且更容易获得。本文报道了镍催化的发展,用于芳基卤化物、Co 2 (CO) 8和硝基芳烃在还原条件下生产芳基酰胺的第一个三组分反应。多种(杂)芳基碘化物和溴化物以及硝基(杂)芳烃是合适的反应伙伴,并且已经实现了高官能团相容性。该方法可用于芳基酰胺的简化合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号