当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Copper-catalyzed coupling of oxime acetates and aryldiazonium salts: an azide-free strategy toward N-2-aryl-1,2,3-triazoles
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-10-25 00:00:00 , DOI: 10.1039/c7qo00874k Chuanle Zhu 1, 2, 3, 4, 5 , Hao Zeng 1, 2, 3, 4, 5 , Fulin Chen 1, 2, 3, 4, 5 , Chi Liu 1, 2, 3, 4, 5 , Rui Zhu 1, 2, 3, 4, 5 , Wanqing Wu 1, 2, 3, 4, 5 , Huanfeng Jiang 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-10-25 00:00:00 , DOI: 10.1039/c7qo00874k Chuanle Zhu 1, 2, 3, 4, 5 , Hao Zeng 1, 2, 3, 4, 5 , Fulin Chen 1, 2, 3, 4, 5 , Chi Liu 1, 2, 3, 4, 5 , Rui Zhu 1, 2, 3, 4, 5 , Wanqing Wu 1, 2, 3, 4, 5 , Huanfeng Jiang 1, 2, 3, 4, 5
Affiliation
|
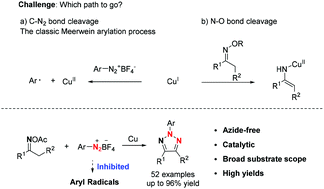
An azide-free strategy for the synthesis of N-2-aryl-1,2,3-triazoles via copper-catalyzed coupling of oxime acetates and aryldiazonium tetrafluoroborates is reported. With the magic p-methoxyphenyldiazonium tetrafluoroborate as the reaction partner, this copper-catalyzed system favored the desired single electron transfer process via N–O bond cleavage of oxime acetates, while the classic single electron transfer process via C–N2 bond cleavage of aryldiazonium tetrafluoroborates was sufficiently inhibited. Significantly, various oxime acetates were suitable for this reaction, delivering the corresponding 4-substituted-N-2-aryl-1,2,3-triazoles and 4,5-disubstituted-N-2-aryl-1,2,3-triazoles in high yield. Primary mechanism studies indicated that this reaction involved a radical procedure. Furthermore, various novel and attractive tetraphenylethylene and carbazole based N-2-aryl-1,2,3-triazoles were synthesized smoothly.
中文翻译:

铜催化的肟肟乙酸盐和芳基重氮盐的偶联:一种针对N -2-芳基-1,2,3-三唑的无叠氮化物策略
用于合成叠氮化物-自由策略Ñ -2-芳基-1,2,3-三唑经由报道肟乙酸酯和芳基重氮四氟硼酸盐的铜催化偶联。以神奇的对氟四氟硼酸对甲氧基苯基重氮作为反应伙伴,该铜催化体系偏向于通过肟肟的N–O键裂解实现所需的单电子转移过程,而经典的单电子通过芳基重氮酮通过C–N 2键裂解进行单电子转移过程。四氟硼酸盐被充分抑制。明显地,各种肟肟适合于该反应,递送相应的4-取代的-N-2-芳基-1,2,3-三唑和4,5-二取代-N -2-芳基-1,2,3-三唑的产率很高。初步机理研究表明,该反应涉及一个基本步骤。此外,顺利地合成了各种新颖且有吸引力的基于四苯基乙烯和咔唑的N -2-芳基-1,2,3-三唑。
更新日期:2017-11-03
中文翻译:

铜催化的肟肟乙酸盐和芳基重氮盐的偶联:一种针对N -2-芳基-1,2,3-三唑的无叠氮化物策略
用于合成叠氮化物-自由策略Ñ -2-芳基-1,2,3-三唑经由报道肟乙酸酯和芳基重氮四氟硼酸盐的铜催化偶联。以神奇的对氟四氟硼酸对甲氧基苯基重氮作为反应伙伴,该铜催化体系偏向于通过肟肟的N–O键裂解实现所需的单电子转移过程,而经典的单电子通过芳基重氮酮通过C–N 2键裂解进行单电子转移过程。四氟硼酸盐被充分抑制。明显地,各种肟肟适合于该反应,递送相应的4-取代的-N-2-芳基-1,2,3-三唑和4,5-二取代-N -2-芳基-1,2,3-三唑的产率很高。初步机理研究表明,该反应涉及一个基本步骤。此外,顺利地合成了各种新颖且有吸引力的基于四苯基乙烯和咔唑的N -2-芳基-1,2,3-三唑。



























 京公网安备 11010802027423号
京公网安备 11010802027423号