当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Site-specific detection of protein secondary structure using 2D IR dihedral indexing: a proposed assembly mechanism of oligomeric hIAPP†
Chemical Science ( IF 7.6 ) Pub Date : 2017-11-03 00:00:00 , DOI: 10.1039/c7sc03789a Michał Maj 1 , Justin P Lomont 1 , Kacie L Rich 1 , Ariel M Alperstein 1 , Martin T Zanni 1
Chemical Science ( IF 7.6 ) Pub Date : 2017-11-03 00:00:00 , DOI: 10.1039/c7sc03789a Michał Maj 1 , Justin P Lomont 1 , Kacie L Rich 1 , Ariel M Alperstein 1 , Martin T Zanni 1
Affiliation
|
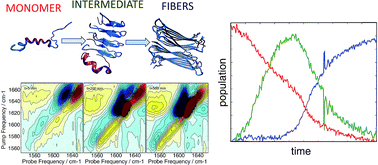
Human islet amyloid polypeptide (hIAPP) aggregates into fibrils through oligomers that have been postulated to contain α-helices as well as β-sheets. We employ a site-specific isotope labeling strategy that is capable of detecting changes in dihedral angles when used in conjunction with 2D IR spectroscopy. The method is analogous to the chemical shift index used in NMR spectroscopy for assigning protein secondary structure. We introduce isotope labels at two neighbouring residues, which results in an increased intensity and positive frequency shift if those residues are α-helical versus a negative frequency shift in β-sheets and turns. The 2D IR dihedral index approach is demonstrated for hIAPP in micelles for which the polypeptide structure is known, using pairs of 13C18O isotope labels L12A13 and L16V17, along with single labeled control experiments. Applying the approach to aggregation experiments performed in buffer, we show that about 27–38% of hIAPP peptides adopt an α-helix secondary structure in the monomeric state at L12A13, prior to aggregation, but not at L16V17 residues. At L16V17, the kinetics are described solely by the monomer and fiber conformations, but at L12A13 the kinetics exhibit a third state that is created by an oligomeric intermediate. Control experiments performed with a single isotope label at A13 exhibit two-state kinetics, indicating that a previously unknown change in dihedral angle occurs at L12A13 as hIAPP transitions from the intermediate to fiber structures. We propose a mechanism for aggregation, in which helices seed oligomer formation via structures analogous to leucine rich repeat proteins.
中文翻译:

使用 2D IR 二面体索引对蛋白质二级结构进行位点特异性检测:寡聚 hIAPP 的拟议组装机制†
人胰岛淀粉样蛋白多肽 (hIAPP) 通过寡聚体聚集成原纤维,推测寡聚体含有 α 螺旋和 β 折叠。我们采用位点特异性同位素标记策略,与二维红外光谱结合使用时能够检测二面角的变化。该方法类似于核磁共振波谱中用于指定蛋白质二级结构的化学位移指数。我们在两个相邻残基处引入同位素标记,如果这些残基是 α 螺旋,则会导致强度增加和正频移,而β 折叠和转角则为负频移。使用成对的13 C 18 O 同位素标记 L12A13 和 L16V17 以及单标记对照实验,针对多肽结构已知的胶束中的 hIAPP 演示了 2D IR 二面体指数方法。应用该方法在缓冲液中进行聚集实验,我们发现约 27-38% 的 hIAPP 肽在聚集之前在 L12A13 处采用单体状态的 α 螺旋二级结构,但在 L16V17 残基处则不然。在 L16V17 中,动力学仅由单体和纤维构象描述,但在 L12A13 中,动力学表现出由低聚中间体产生的第三种状态。在 A13 处使用单一同位素标记进行的对照实验表现出双态动力学,表明当 hIAPP 从中间结构转变为纤维结构时,L12A13 处发生了先前未知的二面角变化。我们提出了一种聚集机制,其中通过类似于富含亮氨酸的重复蛋白的结构来螺旋种子寡聚体的形成。
更新日期:2017-11-03
中文翻译:

使用 2D IR 二面体索引对蛋白质二级结构进行位点特异性检测:寡聚 hIAPP 的拟议组装机制†
人胰岛淀粉样蛋白多肽 (hIAPP) 通过寡聚体聚集成原纤维,推测寡聚体含有 α 螺旋和 β 折叠。我们采用位点特异性同位素标记策略,与二维红外光谱结合使用时能够检测二面角的变化。该方法类似于核磁共振波谱中用于指定蛋白质二级结构的化学位移指数。我们在两个相邻残基处引入同位素标记,如果这些残基是 α 螺旋,则会导致强度增加和正频移,而β 折叠和转角则为负频移。使用成对的13 C 18 O 同位素标记 L12A13 和 L16V17 以及单标记对照实验,针对多肽结构已知的胶束中的 hIAPP 演示了 2D IR 二面体指数方法。应用该方法在缓冲液中进行聚集实验,我们发现约 27-38% 的 hIAPP 肽在聚集之前在 L12A13 处采用单体状态的 α 螺旋二级结构,但在 L16V17 残基处则不然。在 L16V17 中,动力学仅由单体和纤维构象描述,但在 L12A13 中,动力学表现出由低聚中间体产生的第三种状态。在 A13 处使用单一同位素标记进行的对照实验表现出双态动力学,表明当 hIAPP 从中间结构转变为纤维结构时,L12A13 处发生了先前未知的二面角变化。我们提出了一种聚集机制,其中通过类似于富含亮氨酸的重复蛋白的结构来螺旋种子寡聚体的形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号