当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The harpooning mechanism as evidenced in the oxidation reaction of the Al atom†
Chemical Science ( IF 8.4 ) Pub Date : 2017-11-02 00:00:00 , DOI: 10.1039/c7sc03314a Fangfang Li 1, 2, 3, 4, 5 , Changwu Dong 1, 2, 3, 4, 5 , Jun Chen 1, 2, 3, 4, 5 , Jiaxing Liu 1, 2, 3, 4, 5 , Fengyan Wang 1, 2, 3, 4, 5 , Xin Xu 1, 2, 3, 4, 5
Chemical Science ( IF 8.4 ) Pub Date : 2017-11-02 00:00:00 , DOI: 10.1039/c7sc03314a Fangfang Li 1, 2, 3, 4, 5 , Changwu Dong 1, 2, 3, 4, 5 , Jun Chen 1, 2, 3, 4, 5 , Jiaxing Liu 1, 2, 3, 4, 5 , Fengyan Wang 1, 2, 3, 4, 5 , Xin Xu 1, 2, 3, 4, 5
Affiliation
|
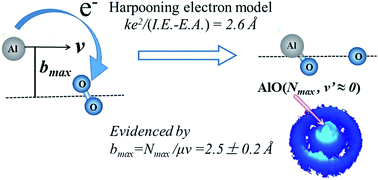
The harpooning mechanism has long been proposed for elementary reaction dynamics involving metals. It is characterized by an initial electron transfer (ET) process from the metal to the oxidant molecule. For the titled reaction Al + O2, the ET distance can be predicted to be 2.6 Å by simply calculating the energy difference between the ionization energy of the Al atom and the electron affinity of the O2 molecule. Hereby we experimentally derived the maximum impact parameter bmax of 2.5 ± 0.2 Å for the titled reaction, in consistency with the predicted ET distance. This derivation of bmax was achieved by using the crossed molecular beam experiment at a collision energy of 507 cm−1 (i.e. 1.45 kcal mol−1) with a high resolution time-sliced ion velocity imaging detection of the state-selective AlO products based on the (1 + 1) resonance-enhanced multiphoton ionization. The small rotational constant of the AlO(X2Σ+) radical (Be = 0.6413 cm−1) facilitated the formation of the AlO(v = 0) products in high rotational levels up to the energetically limited state, Nmax = 52, with an almost zero velocity mapping. Hence, in this extreme angular momentum disposal case, the collisional orbital angular momentum l was nearly completely channeled into the product rotational angular momentum as a consequence of the conservations of energy and angular momentum, offering a reaction system that breaks the restriction of kinematically favored mass combination in order to obtain information on the impact parameters. The present study yields the first direct derivation of bmax from the maximum rotational level of products under the experimental condition with the recoil energy E′T ≈ 0. This, in turn, provides solid evidence in supporting the harpooning mechanism.
中文翻译:

铝原子的氧化反应中所证实的鱼叉机制†
对于涉及金属的基本反应动力学,人们早就提出了鱼叉机制。它的特征是从金属到氧化剂分子的初始电子转移(ET)过程。对于标题为Al + O 2的反应,通过简单地计算Al原子的电离能与O 2分子的电子亲和力之间的能差,可以预测ET距离为2.6 。因此,我们通过实验得出了标题反应的最大冲击参数b max为2.5±0.2Å,与预测的ET距离一致。b max的推导是通过使用交叉分子束实验在507 cm -1(即1.45 kcal mol -1)的高分辨率时间切片离子速度成像检测基于(1 +1)共振增强多光子电离的状态选择AlO产物。所述的AlO的小的旋转常数(X 2 Σ +)基团(乙Ë =0.6413厘米-1)促进了的AlO的形成(v = 0)的产品在高的旋转水平,直至在能量限制状态,Ñ最大= 52 ,速度映射几乎为零。因此,在这种极端角动量处理的情况下,碰撞轨道角动量l由于能量和角动量的守恒,它几乎完全导入了产品旋转角动量中,从而提供了一种反应系统,该运动系统打破了运动学上有利的质量组合的限制,从而获得了有关冲击参数的信息。本研究中产生的所述第一直接推导方法b最大值从产品的最大转动水平与反冲能量在实验条件下è ' Ť ≈0。这,反过来,提供了在支持镖机构坚实的证据。
更新日期:2017-11-02
中文翻译:

铝原子的氧化反应中所证实的鱼叉机制†
对于涉及金属的基本反应动力学,人们早就提出了鱼叉机制。它的特征是从金属到氧化剂分子的初始电子转移(ET)过程。对于标题为Al + O 2的反应,通过简单地计算Al原子的电离能与O 2分子的电子亲和力之间的能差,可以预测ET距离为2.6 。因此,我们通过实验得出了标题反应的最大冲击参数b max为2.5±0.2Å,与预测的ET距离一致。b max的推导是通过使用交叉分子束实验在507 cm -1(即1.45 kcal mol -1)的高分辨率时间切片离子速度成像检测基于(1 +1)共振增强多光子电离的状态选择AlO产物。所述的AlO的小的旋转常数(X 2 Σ +)基团(乙Ë =0.6413厘米-1)促进了的AlO的形成(v = 0)的产品在高的旋转水平,直至在能量限制状态,Ñ最大= 52 ,速度映射几乎为零。因此,在这种极端角动量处理的情况下,碰撞轨道角动量l由于能量和角动量的守恒,它几乎完全导入了产品旋转角动量中,从而提供了一种反应系统,该运动系统打破了运动学上有利的质量组合的限制,从而获得了有关冲击参数的信息。本研究中产生的所述第一直接推导方法b最大值从产品的最大转动水平与反冲能量在实验条件下è ' Ť ≈0。这,反过来,提供了在支持镖机构坚实的证据。



























 京公网安备 11010802027423号
京公网安备 11010802027423号