当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Towards a Step-Economical and Waste-Free [hmim]Br-Catalyzed Deprotection of β-Sulfido Carbonyl Groups into (E)-Enones and Mechanistic Insights
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-11-02 02:23:40 , DOI: 10.1002/ajoc.201700409 Yogesh Thopate 1, 2 , Richa Singh 1 , Tanuj Sharma 3 , Mohammad I. Siddiqi 3 , Arun K. Sinha 1, 2
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-11-02 02:23:40 , DOI: 10.1002/ajoc.201700409 Yogesh Thopate 1, 2 , Richa Singh 1 , Tanuj Sharma 3 , Mohammad I. Siddiqi 3 , Arun K. Sinha 1, 2
Affiliation
|
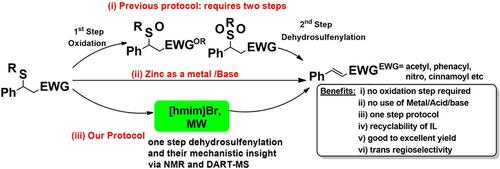
Waste not, want not: The direct deprotection of β-sulfido compounds into α,β-unsaturated alkenes has been achieved by using a neutral ionic liquid, [hmim]Br, as a solvent and catalyst. This method is mild, highly regioselective, and offers broad substrate scope and high yields, as well as recyclability of the ionic liquid for up to five cycles. 1H NMR and MS (DART) analysis provided mechanistic insight into the role of [hmim]Br in the reaction.
中文翻译:

逐步实现经济,无浪费的[hmim] Br催化的β-硫代羰基脱保护成(E)-酮和机理的研究
不要浪费,不要浪费:通过使用中性离子液体[hmim] Br作为溶剂和催化剂,可以将β-硫键化合物直接脱保护为α,β-不饱和烯烃。该方法是温和的,对区域具有高选择性的,并提供了广泛的底物范围和高收率,以及离子液体最多可循环使用五个周期。1 H NMR和MS(DART)分析为[hmim] Br在反应中的作用提供了机理上的见解。
更新日期:2017-11-03
中文翻译:

逐步实现经济,无浪费的[hmim] Br催化的β-硫代羰基脱保护成(E)-酮和机理的研究
不要浪费,不要浪费:通过使用中性离子液体[hmim] Br作为溶剂和催化剂,可以将β-硫键化合物直接脱保护为α,β-不饱和烯烃。该方法是温和的,对区域具有高选择性的,并提供了广泛的底物范围和高收率,以及离子液体最多可循环使用五个周期。1 H NMR和MS(DART)分析为[hmim] Br在反应中的作用提供了机理上的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号