当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic Investigation into RhIII‐Catalyzed Intramolecular Redox‐Neutral Annulation of Aryl Hydrazines with a Tethered Alkyne
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2017-11-02 , DOI: 10.1002/ajoc.201700469 Weirong Wu 1 , Dongcheng Ren 1 , Benzhen Xu 1 , Xiaoying Ma 1 , Caiyun Huang 1 , Jing Zhang 1 , Tao Liu 1, 2
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2017-11-02 , DOI: 10.1002/ajoc.201700469 Weirong Wu 1 , Dongcheng Ren 1 , Benzhen Xu 1 , Xiaoying Ma 1 , Caiyun Huang 1 , Jing Zhang 1 , Tao Liu 1, 2
Affiliation
|
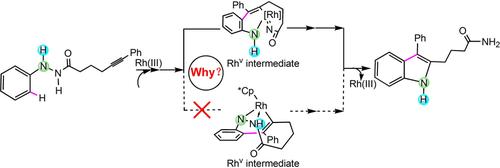
A mechanistic study of the Cp*RhIII‐catalyzed (Cp*=η5‐pentamethylcyclopentadienyl) intramolecular redox‐neutral annulation of tethered alkynes was carried out by density functional calculations using the M06 method. Our results show that the reductive elimination step of the catalytic cycle is rate determining, and the overall energy barrier of the entire process is 31.4 kcal mol−1 (L‐II→L‐TS8‐9). This is in agreement with experimental results that reported the C−H bond cleavage not to be the rate‐determining step. According to our calculations, the feasible mechanism for the RhIII→RhV→RhIII process involves an acylamino migration and subsequent reductive elimination process, which is different from the pathway previously proposed by Li's group. The present calculations elucidate the experimental observations on the molecular level.
中文翻译:

RhIII催化的带有链状炔烃的芳族肼的分子内氧化还原中性环化的机理研究
通过使用M06方法进行密度泛函计算,对Cp * Rh III催化的(Cp * =η5-五甲基甲基环戊二烯基)分子内氧化还原中性束缚炔烃的机理进行了研究。我们的结果表明,该催化循环的还原消除步骤是速率确定,并且整个过程的总能量势垒是31.4千卡摩尔-1(L-II → L-TS8 - 9)。这与实验结果一致,后者报告的CH键断裂不是决定速率的步骤。根据我们的计算,Rh III →Rh V →Rh III的可行机理这一过程涉及酰基氨基迁移和随后的还原消除过程,这与李氏团队先前提出的途径不同。目前的计算阐明了在分子水平上的实验观察。
更新日期:2017-11-02
中文翻译:

RhIII催化的带有链状炔烃的芳族肼的分子内氧化还原中性环化的机理研究
通过使用M06方法进行密度泛函计算,对Cp * Rh III催化的(Cp * =η5-五甲基甲基环戊二烯基)分子内氧化还原中性束缚炔烃的机理进行了研究。我们的结果表明,该催化循环的还原消除步骤是速率确定,并且整个过程的总能量势垒是31.4千卡摩尔-1(L-II → L-TS8 - 9)。这与实验结果一致,后者报告的CH键断裂不是决定速率的步骤。根据我们的计算,Rh III →Rh V →Rh III的可行机理这一过程涉及酰基氨基迁移和随后的还原消除过程,这与李氏团队先前提出的途径不同。目前的计算阐明了在分子水平上的实验观察。



























 京公网安备 11010802027423号
京公网安备 11010802027423号