当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactions involving some gas molecules through sequestration on Al12 Be cluster: An electron density based study
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2017-11-01 , DOI: 10.1002/jcc.25092 Debdutta Chakraborty 1 , Pratim Kumar Chattaraj 1
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2017-11-01 , DOI: 10.1002/jcc.25092 Debdutta Chakraborty 1 , Pratim Kumar Chattaraj 1
Affiliation
|
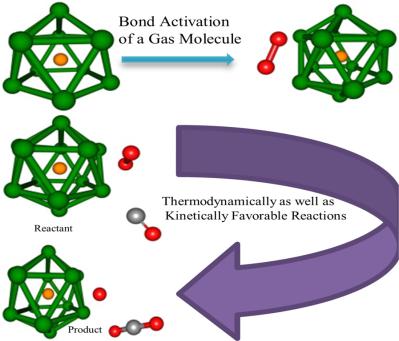
The viability of sequestering gas molecules (CO, NO, CO2, NO2, N2O, O2, O3, H2O, NH3, H2, CH3OH, CH3F, C2H5F, C2H2, C2H4, HCN, and SO2) on the Al12Be cluster is investigated by carrying out density functional theory based computations. Thermochemical as well as energetic considerations suggest that Al12Be cluster adsorbs the chosen gas molecules in a favorable fashion. The gas molecules attain an activated state on getting adsorbed on the metal cluster as vindicated by Atoms‐in‐Molecule analysis. The possibility of CO oxidation, dissociative addition of CH3F and C2H5F, NH bond decomposition in NH3, dissociation of NO, and hydrogenation of C2H2 reactions on Al12Be cluster has been investigated. Results indicate that all the reactions take place in a thermodynamically favorable way at 298.15 K and one atmospheric pressure. The first five reactions aforementioned are kinetically favorable also and therefore are amenable to ambient temperature and pressure conditions. © 2017 Wiley Periodicals, Inc.
中文翻译:

通过螯合 Al12 Be 团簇而涉及一些气体分子的反应:基于电子密度的研究
Al12Be 簇上隔离气体分子(CO、NO、CO2、NO2、N2O、O2、O3、H2O、NH3、H2、CH3OH、CH3F、C2H5F、C2H2、C2H4、HCN 和 SO2)的可行性通过携带基于密度泛函理论的计算。热化学和能量考虑表明 Al12Be 簇以有利的方式吸附所选的气体分子。原子分子分析表明,气体分子在吸附在金属簇上时达到活化状态。已经研究了 CO 氧化、CH3F 和 C2H5F 解离加成、NH3 中 NH 键分解、NO 解离和 C2H2 加氢反应在 Al12Be 簇上的可能性。结果表明,所有反应都在 298.15 K 和一个大气压下以热力学有利的方式发生。上述前五个反应在动力学上也是有利的,因此适用于环境温度和压力条件。© 2017 威利期刊公司。
更新日期:2017-11-01
中文翻译:

通过螯合 Al12 Be 团簇而涉及一些气体分子的反应:基于电子密度的研究
Al12Be 簇上隔离气体分子(CO、NO、CO2、NO2、N2O、O2、O3、H2O、NH3、H2、CH3OH、CH3F、C2H5F、C2H2、C2H4、HCN 和 SO2)的可行性通过携带基于密度泛函理论的计算。热化学和能量考虑表明 Al12Be 簇以有利的方式吸附所选的气体分子。原子分子分析表明,气体分子在吸附在金属簇上时达到活化状态。已经研究了 CO 氧化、CH3F 和 C2H5F 解离加成、NH3 中 NH 键分解、NO 解离和 C2H2 加氢反应在 Al12Be 簇上的可能性。结果表明,所有反应都在 298.15 K 和一个大气压下以热力学有利的方式发生。上述前五个反应在动力学上也是有利的,因此适用于环境温度和压力条件。© 2017 威利期刊公司。











































 京公网安备 11010802027423号
京公网安备 11010802027423号