当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective Deuteration in Aspartate, Asparagine, Lysine, and Methionine Amino Acid Residues Using Fumarate as a Carbon Source for Escherichia coli in D2O
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-03 00:00:00 , DOI: 10.1021/acs.biochem.7b00991 Gaddafi I. Danmaliki 1 , Philip B. Liu 2 , Peter M. Hwang 1, 2
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-03 00:00:00 , DOI: 10.1021/acs.biochem.7b00991 Gaddafi I. Danmaliki 1 , Philip B. Liu 2 , Peter M. Hwang 1, 2
Affiliation
|
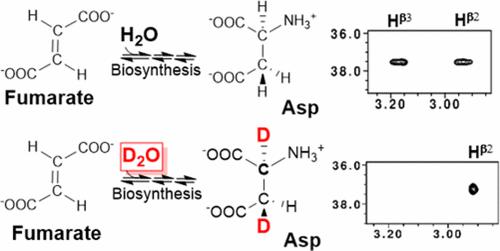
Perdeuteration with selective 1H,13C-enrichment of methyl groups has enabled solution NMR studies of large (>30 kDa) protein systems. However, we propose that for all non-methyl positions, only magnetization originating from 1H–12C groups is sufficiently long-lived, and it can be transferred via through-space NOEs to slowly relaxing 1H–15N or 1H–13C methyl groups to achieve multidimensional solution NMR. We demonstrate stereoselective 1H,12C-labeling by adding relatively inexpensive unlabeled carbon sources to Escherichia coli growth media in D2O. Using our model system, a mutant WW domain from human Pin1, we compare deuteration patterns in 19 amino acids (all except cysteine). Protein grown using glucose as the sole carbon source had high levels of protonation in aromatic rings and the Hβ positions of serine and tryptophan. In contrast, using our FROMP media (fumarate, rhamnose, oxalate, malonate, pyruvate), stereoselective protonation of Hβ2 with deuteration at Hα and Hβ3 was achieved in Asp, Asn, Lys, and Met residues. In solution NMR, stereospecific chemical shift assignments for Hβ are typically obtained in conjunction with χ1 dihedral angle determinations using 3-bond J-coupling (3JN–Hβ, 3JCO-Hβ, 3JHα-Hβ) experiments. However, due to motional averaging, the assumption of a pure rotameric state can yield incorrect χ1 dihedral angles with incorrect stereospecific assignments. This was the case for three residues in the Pin1 WW domain (Lys28, Met30, and Asn44). Thus, stereoselective 1H,12C-labeling will be useful not only for NMR studies of large protein systems, but also for determining side chain rotamers and dynamics in any protein system.
中文翻译:

使用富马酸酯作为D 2 O中大肠杆菌的碳源,在天冬氨酸,天冬酰胺,赖氨酸和蛋氨酸氨基酸残基中进行立体选择性氘化
具有选择性的1 H,13 C甲基富集的氘代化使大型(> 30 kDa)蛋白质系统的溶液NMR研究成为可能。但是,我们建议,对于所有非甲基位置,仅源自1 H– 12 C基团的磁化寿命足够长,并且可以通过空间NOE转移至缓慢弛豫1 H– 15 N或1 H–实现13 C甲基的多维溶液NMR。我们通过向D中的大肠杆菌生长培养基中添加相对便宜的未标记碳源来证明立体选择性1 H,12 C标记2 O.使用我们的模型系统(来自人Pin1的突变WW域),我们比较了19个氨基酸(除半胱氨酸以外的所有氨基酸)的氘代模式。蛋白快把使用葡萄糖作为唯一碳源有在芳香环高水平质子化和H β丝氨酸和色氨酸的位置。与此相反,使用我们FROMP媒体(富马酸盐,鼠李糖,草酸盐,丙二酸盐,丙酮酸盐),H的立体选择性质子化β2与处于H氘α和H β3在天冬氨酸,天冬酰胺,赖氨酸实现,和Met的残基。在溶液NMR,用于h立体特异性的化学位移分配β通常结合χ获得1所使用3-键二面角确定Ĵ-耦合(3 Ĵ N-二Hβ,3 Ĵ CO-Hβ,3 Ĵ Hα-Hβ)的实验。然而,由于运动平均,一个纯粹的旋转异构体状态的假设可能产生不正确的χ 1不正确的立体有择的分配二面角。Pin1 WW域中的三个残基(Lys28,Met30和Asn44)就是这种情况。因此,立体选择性1 H,12 C标记不仅可用于大型蛋白质系统的NMR研究,而且还可用于确定任何蛋白质系统中的侧链旋转异构体和动力学。
更新日期:2017-11-03
中文翻译:

使用富马酸酯作为D 2 O中大肠杆菌的碳源,在天冬氨酸,天冬酰胺,赖氨酸和蛋氨酸氨基酸残基中进行立体选择性氘化
具有选择性的1 H,13 C甲基富集的氘代化使大型(> 30 kDa)蛋白质系统的溶液NMR研究成为可能。但是,我们建议,对于所有非甲基位置,仅源自1 H– 12 C基团的磁化寿命足够长,并且可以通过空间NOE转移至缓慢弛豫1 H– 15 N或1 H–实现13 C甲基的多维溶液NMR。我们通过向D中的大肠杆菌生长培养基中添加相对便宜的未标记碳源来证明立体选择性1 H,12 C标记2 O.使用我们的模型系统(来自人Pin1的突变WW域),我们比较了19个氨基酸(除半胱氨酸以外的所有氨基酸)的氘代模式。蛋白快把使用葡萄糖作为唯一碳源有在芳香环高水平质子化和H β丝氨酸和色氨酸的位置。与此相反,使用我们FROMP媒体(富马酸盐,鼠李糖,草酸盐,丙二酸盐,丙酮酸盐),H的立体选择性质子化β2与处于H氘α和H β3在天冬氨酸,天冬酰胺,赖氨酸实现,和Met的残基。在溶液NMR,用于h立体特异性的化学位移分配β通常结合χ获得1所使用3-键二面角确定Ĵ-耦合(3 Ĵ N-二Hβ,3 Ĵ CO-Hβ,3 Ĵ Hα-Hβ)的实验。然而,由于运动平均,一个纯粹的旋转异构体状态的假设可能产生不正确的χ 1不正确的立体有择的分配二面角。Pin1 WW域中的三个残基(Lys28,Met30和Asn44)就是这种情况。因此,立体选择性1 H,12 C标记不仅可用于大型蛋白质系统的NMR研究,而且还可用于确定任何蛋白质系统中的侧链旋转异构体和动力学。









































 京公网安备 11010802027423号
京公网安备 11010802027423号