当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reductive Alkylation of Tertiary Lactams via Addition of Organocopper (RCu) Reagents to Thioiminium Ions
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-02 00:00:00 , DOI: 10.1021/acs.joc.7b02150 Pierre Mateo 1 , Joséphine E. Cinqualbre 1 , Melinda Meyer Mojzes 1 , Kurt Schenk 2 , Philippe Renaud 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-02 00:00:00 , DOI: 10.1021/acs.joc.7b02150 Pierre Mateo 1 , Joséphine E. Cinqualbre 1 , Melinda Meyer Mojzes 1 , Kurt Schenk 2 , Philippe Renaud 1
Affiliation
|
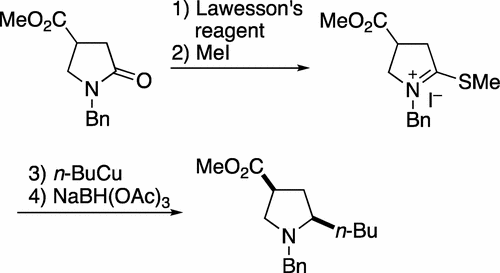
A simple procedure for the conversion of tertiary lactams to 2-monoalkylated cyclic amines is described. The reaction sequence involves conversion of a lactam to a thioiminium ion followed by reaction with an organocopper (RCu) reagent and final reduction with triacetoxyborohydride. The reaction is high yielding and shows an excellent functional group tolerance. Its utility is demonstrated by a rapid synthesis of indolizidine 167B. The excellent chemoselectivity of the process, where only monoalkylation products are formed, is rationalized by a mechanism involving the formation of a transient enamine.
中文翻译:

通过将有机铜(RCu)试剂添加到硫代亚胺离子上来还原内酰胺的烷基化反应
描述了将叔内酰胺转化为2-单烷基化的环胺的简单程序。反应顺序包括将内酰胺转化为硫亚胺离子,然后与有机铜(RCu)试剂反应,最后用三乙酰氧基硼氢化物还原。该反应产率高并且显示出优异的官能团耐受性。吲哚并立定167B的快速合成证明了其实用性。仅形成单烷基化产物的该方法的优异化学选择性通过涉及形成瞬时烯胺的机理来合理化。
更新日期:2017-11-03
中文翻译:

通过将有机铜(RCu)试剂添加到硫代亚胺离子上来还原内酰胺的烷基化反应
描述了将叔内酰胺转化为2-单烷基化的环胺的简单程序。反应顺序包括将内酰胺转化为硫亚胺离子,然后与有机铜(RCu)试剂反应,最后用三乙酰氧基硼氢化物还原。该反应产率高并且显示出优异的官能团耐受性。吲哚并立定167B的快速合成证明了其实用性。仅形成单烷基化产物的该方法的优异化学选择性通过涉及形成瞬时烯胺的机理来合理化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号