Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2017-11-01 , DOI: 10.1016/j.apcata.2017.10.022 Lin Liu , Tatsumi Ishihara
|
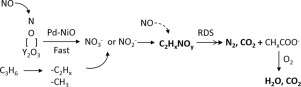
Y(Ba)2O3 catalyst loaded with 1 wt% Pd and 3.2 wt% NiO, Pd-NiO/(Y0.99Ba0.01)2O3, was found to be highly active to the selective reduction of NO with C3H6. The adsorption of NO on both (Y0.99Ba0.01)2O3 and Pd-NiO loaded one was studied using temperature-programmed desorption, pulsed reaction analysis and in-situ Fourier transform infrared (FTIR) spectroscopy. It was determined that NO is readily adsorbed on (Y0.99Ba0.01)2O3, primarily via the formation of nitrite and nitrate species. The addition of Pd-NiO assists in activating NO decomposition at lower temperatures as well as increasing the extent of NO adsorption. The reaction of NO with C3H6 and O2 on the Pd-NiO/(Y0.99Ba0.01)2O3 was also studied using pulsed reaction analysis and in-situ FTIR under NO selective reduction condition. The results demonstrated that the mechanism involves the reaction of −C2Hx or −CH3 adsorption species generated via the disproportionation of C3H6 with nitrate species to form CNO or C2HxNOy (x, y = 2,3) intermediates. These intermediates readily react with NO and O2 to form the products CO2, N2 and H2O.
中文翻译:

Pd-NiO /(Y 0.99 Ba 0.01)2 O 3催化剂选择性还原NO的还原机理
发现载有1 wt%Pd和3.2 wt%NiO,Pd-NiO /(Y 0.99 Ba 0.01)2 O 3的Y(Ba)2 O 3催化剂对用C 3 H选择性还原NO具有很高的活性。6。使用程序升温脱附,脉冲反应分析和原位傅里叶变换红外光谱研究了在(Y 0.99 Ba 0.01)2 O 3和Pd-NiO上NO的吸附。确定NO易于吸附在(Y 0.99 Ba 0.01)2 O 3上,主要是通过亚硝酸盐和硝酸盐物种的形成。Pd-NiO的添加有助于在较低温度下激活NO分解,并增加NO的吸附程度。还利用脉冲反应分析和原位FTIR在NO选择性还原条件下研究了NO与C 3 H 6和O 2在Pd-NiO /(Y 0.99 Ba 0.01)2 O 3上的反应。结果表明,该机理涉及通过C 3 H 6歧化产生的-C 2 H x或-CH 3吸附物种的反应。与硝酸盐物质形成CNO或C 2 H x NO y(x,y = 2,3)中间体。这些中间体容易与NO和O反应2以形成所述产品CO 2,N 2和H 2 O.











































 京公网安备 11010802027423号
京公网安备 11010802027423号