当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Chemical Proteomics Approach to Reveal Direct Protein-Protein Interactions in Living Cells
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2017-11-02 , DOI: 10.1016/j.chembiol.2017.10.001 Ralph E. Kleiner , Lisa E. Hang , Kelly R. Molloy , Brian T. Chait , Tarun M. Kapoor
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2017-11-02 , DOI: 10.1016/j.chembiol.2017.10.001 Ralph E. Kleiner , Lisa E. Hang , Kelly R. Molloy , Brian T. Chait , Tarun M. Kapoor
|
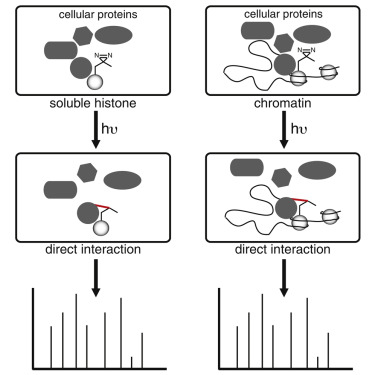
Protein-protein interactions mediate essential cellular processes, however the detection of native interactions is challenging since they are often low affinity and context dependent. Here, we develop a chemical proteomics approach in vivo CLASPI [iCLASPI] (in vivocrosslinking-assisted and stable isotope labeling by amino acids in cell culture [SILAC]-based protein identification) relying upon photo-crosslinking, amber suppression, and SILAC-based quantitative proteomics to profile context-dependent protein-protein interactions in living cells. First, we use iCLASPI to profilein vivobinding partners of the N-terminal tails of soluble histone H3 or H4. We identify known histone chaperones and modifying proteins, thereby validating our approach, and find an interaction between soluble histone H3 and UBR7, an E3 ubiquitin ligase, mediated by UBR7's PHD domain. Furthermore, we apply iCLASPI to profile the context-dependent protein-protein interactions of chromatin-associated histone H3 at different cell-cycle stages, and identify ANP32A as a mitosis-specific interactor. Our results demonstrate that the iCLASPI approach can provide a general strategy for identifying native, context-dependent direct protein-protein interactions using photo-crosslinking and quantitative proteomics.
中文翻译:

化学蛋白质组学方法揭示活细胞中直接蛋白质-蛋白质相互作用
蛋白质-蛋白质相互作用介导基本的细胞过程,但是天然相互作用的检测具有挑战性,因为它们通常是低亲和力和背景依赖性的。在这里,我们依靠光交联,琥珀色抑制和基于SILAC的方法,开发了一种基于体内CLASPI [iCLASPI]的化学蛋白质组学方法(通过细胞培养中基于氨基酸的体内交联辅助和稳定的同位素标记[SILAC]进行蛋白质鉴定)。定量蛋白质组学来描述活细胞中上下文相关的蛋白质-蛋白质相互作用。首先,我们使用iCLASPI来分析可溶性组蛋白H3或H4的N末端尾巴的体内结合伴侣。我们鉴定出已知的组蛋白伴侣和修饰蛋白,从而验证了我们的方法,并发现了可溶性组蛋白H3与UBR7(一种由UBR7'介导的E3泛素连接酶)之间的相互作用。的PHD域。此外,我们应用iCLASPI来分析与染色质相关的组蛋白H3在不同细胞周期阶段的上下文相关的蛋白质-蛋白质相互作用,并将ANP32A鉴定为有丝分裂特异性相互作用子。我们的研究结果表明,iCLASPI方法可提供一种使用光交联和定量蛋白质组学鉴定天然的,上下文相关的直接蛋白质-蛋白质相互作用的一般策略。
更新日期:2018-01-18
中文翻译:

化学蛋白质组学方法揭示活细胞中直接蛋白质-蛋白质相互作用
蛋白质-蛋白质相互作用介导基本的细胞过程,但是天然相互作用的检测具有挑战性,因为它们通常是低亲和力和背景依赖性的。在这里,我们依靠光交联,琥珀色抑制和基于SILAC的方法,开发了一种基于体内CLASPI [iCLASPI]的化学蛋白质组学方法(通过细胞培养中基于氨基酸的体内交联辅助和稳定的同位素标记[SILAC]进行蛋白质鉴定)。定量蛋白质组学来描述活细胞中上下文相关的蛋白质-蛋白质相互作用。首先,我们使用iCLASPI来分析可溶性组蛋白H3或H4的N末端尾巴的体内结合伴侣。我们鉴定出已知的组蛋白伴侣和修饰蛋白,从而验证了我们的方法,并发现了可溶性组蛋白H3与UBR7(一种由UBR7'介导的E3泛素连接酶)之间的相互作用。的PHD域。此外,我们应用iCLASPI来分析与染色质相关的组蛋白H3在不同细胞周期阶段的上下文相关的蛋白质-蛋白质相互作用,并将ANP32A鉴定为有丝分裂特异性相互作用子。我们的研究结果表明,iCLASPI方法可提供一种使用光交联和定量蛋白质组学鉴定天然的,上下文相关的直接蛋白质-蛋白质相互作用的一般策略。









































 京公网安备 11010802027423号
京公网安备 11010802027423号