当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Poly(methyl 6-acryloyl-β-d-glucosaminoside) as a Cationic Glycomimetic of Chitosan
Biomacromolecules ( IF 5.5 ) Pub Date : 2017-11-01 00:00:00 , DOI: 10.1021/acs.biomac.7b01191 Walter T. Liau 1 , Andrea M. Kasko 1
Biomacromolecules ( IF 5.5 ) Pub Date : 2017-11-01 00:00:00 , DOI: 10.1021/acs.biomac.7b01191 Walter T. Liau 1 , Andrea M. Kasko 1
Affiliation
|
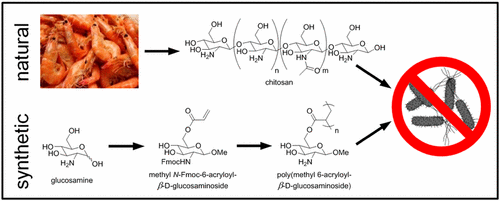
Chitosan, a cationic polysaccharide derived from one of the most abundant natural polymers, chitin, has been investigated extensively for its antimicrobial properties. However, it suffers from the inherent drawbacks of natural products such as batch-to-batch variability, limited supply, contamination, and potential adverse reaction. Additionally, its solubility depends on the degree of deacetylation and pH, as it is only soluble under acidic conditions. As an alternative to chitosan, we synthesized the protected cationic glycomimetic monomer methyl N-Fmoc-6-acryloyl-β-d-glucosaminoside from glucosamine. This monomer retains structural features critical to recapitulating the properties of the chitosan repeat unit, namely, the pKa of the protonated amine. We optimized the free radical polymerization of methyl N-Fmoc-6-acryloyl-β-d-glucosaminoside and fractionated the resultant poly(methyl N-Fmoc-6-acryloyl-β-d-glucosaminoside) to obtain a range of molecular weights. Following Fmoc deprotection, the cationic glycopolymers retained 95% of their expected amine content by mass and exhibited a pKa of 6.61. Poly(methyl 6-acryloyl-β-d-glucosaminoside) mimicked the molecular weight-dependent bacterial inhibitory property of chitosan in acidic solutions. Importantly, poly(methyl 6-acryloyl-β-d-glucosaminoside) remained soluble at elevated pH (conditions under which chitosan is insoluble) and maintained its antibacterial activity. Mammalian cell viability in the presence of poly(methyl 6-acryloyl-β-d-glucosaminoside) at acidic pH is good, although somewhat lower than viability in the presence of chitosan. No cytotoxic effect was observed at neutral pH. These results demonstrate that poly(methyl 6-acryloyl-β-d-glucosaminoside) is not only a suitable biomimetic for chitosan, but that it can be utilized as an antibacterial agent in a broader range of biologically relevant pHs.
中文翻译:

聚(甲基6-丙烯酰基-β-d-葡糖氨基糖苷)作为壳聚糖的阳离子仿制药
壳聚糖是一种衍生自最丰富的天然聚合物几丁质的阳离子多糖,其抗菌性能已得到广泛研究。但是,它具有天然产物的固有缺点,例如批次间的可变性,有限的供应,污染和潜在的不良反应。另外,它的溶解度取决于脱乙酰基的程度和pH,因为它仅在酸性条件下可溶。作为壳聚糖的替代品,我们从葡糖胺合成了被保护的阳离子模拟糖单体甲基N -Fmoc-6-丙烯酰基-β - d-葡糖氨基糖苷。该单体保留了对壳聚糖重复单元的性能概括至关重要的结构特征,即p K a质子化胺。我们优化了甲基N -Fmoc-6-丙烯酰基-β - d-葡糖氨基糖苷的自由基聚合反应,并将所得的聚(甲基N - Fmoc -6-丙烯酰基-β - d-葡糖氨基糖苷)分馏以获得一定范围的分子量。Fmoc脱保护后,阳离子糖聚合物按质量计保留了其预期胺含量的95%,并且ap K a为6.61。聚(甲基6-丙烯酰基-β - d-葡糖氨基糖苷)模仿了壳聚糖在酸性溶液中的分子量依赖性细菌抑制特性。重要的是,聚(甲基6-丙烯酰基-β- d-葡糖氨基糖苷)在升高的pH值(壳聚糖不溶的条件下)保持可溶,并保持其抗菌活性。在聚(甲基6-丙烯酰基-β - d-葡糖氨基糖苷)存在下,在酸性pH条件下,哺乳动物细胞的存活力良好,尽管比在壳聚糖存在下的存活力低。在中性pH下未观察到细胞毒性作用。这些结果表明,聚(甲基6-丙烯酰基-β - d-葡糖氨基糖苷)不仅是壳聚糖的合适仿生剂,而且可以在更广泛的生物学相关pH中用作抗菌剂。
更新日期:2017-11-01
中文翻译:

聚(甲基6-丙烯酰基-β-d-葡糖氨基糖苷)作为壳聚糖的阳离子仿制药
壳聚糖是一种衍生自最丰富的天然聚合物几丁质的阳离子多糖,其抗菌性能已得到广泛研究。但是,它具有天然产物的固有缺点,例如批次间的可变性,有限的供应,污染和潜在的不良反应。另外,它的溶解度取决于脱乙酰基的程度和pH,因为它仅在酸性条件下可溶。作为壳聚糖的替代品,我们从葡糖胺合成了被保护的阳离子模拟糖单体甲基N -Fmoc-6-丙烯酰基-β - d-葡糖氨基糖苷。该单体保留了对壳聚糖重复单元的性能概括至关重要的结构特征,即p K a质子化胺。我们优化了甲基N -Fmoc-6-丙烯酰基-β - d-葡糖氨基糖苷的自由基聚合反应,并将所得的聚(甲基N - Fmoc -6-丙烯酰基-β - d-葡糖氨基糖苷)分馏以获得一定范围的分子量。Fmoc脱保护后,阳离子糖聚合物按质量计保留了其预期胺含量的95%,并且ap K a为6.61。聚(甲基6-丙烯酰基-β - d-葡糖氨基糖苷)模仿了壳聚糖在酸性溶液中的分子量依赖性细菌抑制特性。重要的是,聚(甲基6-丙烯酰基-β- d-葡糖氨基糖苷)在升高的pH值(壳聚糖不溶的条件下)保持可溶,并保持其抗菌活性。在聚(甲基6-丙烯酰基-β - d-葡糖氨基糖苷)存在下,在酸性pH条件下,哺乳动物细胞的存活力良好,尽管比在壳聚糖存在下的存活力低。在中性pH下未观察到细胞毒性作用。这些结果表明,聚(甲基6-丙烯酰基-β - d-葡糖氨基糖苷)不仅是壳聚糖的合适仿生剂,而且可以在更广泛的生物学相关pH中用作抗菌剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号