Catalysis Today ( IF 5.3 ) Pub Date : 2017-10-31 , DOI: 10.1016/j.cattod.2017.10.047 Syuhei Yamaguchi , Yuki Miyake , Keiko Takiguchi , Daijiro Ihara , Hidenori Yahiro
|
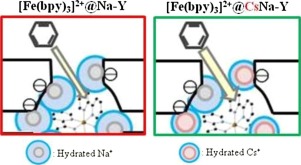
Fe-bipyridine complexes were encapsulated into cation-exchanged Y-type zeolites (M-Na-Y: M = K+, Cs+, Mg2+, Ca2+, NH4+, TMA+, and TBA+) and their catalytic activities for oxidation of benzene with hydrogen peroxide (H2O2) to phenol were investigated in three types of solvents (CH3CN, H2O, and CH3CN + H2O(1:1)). Regardless of the kind of solvent, the counter cation in [Fe(bpy)3]2+@M-Na-Y did not affect the selectivity to phenol. No significant difference in catalytic activity of [Fe(bpy)3]2+@M-Na-Y appeared in CH3CN + H2O (1:1), while a difference on catalytic activity appeared in each CH3CN and H2O solvent. It was suggested that the catalytic activity is related with the accessibility of benzene to [Fe(bpy)3]2+ site in [Fe(bpy)3]2+@M-Na-Y, controlled by the hydrated ionic radius of the counter cation introduced. The effect of ligand coordinated with Fe ion was investigated by comparing the catalytic activities for oxidation of cyclic hydrocarbons (benzene, cyclohexane, and cyclohexene) over [Fe(bpy)3]2+@Na-Y, [Fe(phen)3]2+@Na-Y, and [Fe(terpy)2]2+@Na-Y (phen = 1,10-phenanthroline and terpy = 2,2′;6′,2″-terpyridine). It was suggested that the expansion of π-electron over the ligands such as phen and terpy improves the uptake ability of substrates having π-electron such as benzene.
中文翻译:

阳离子交换沸石包裹的铁络合物上用过氧化氢氧化环状烃
Fe-联吡啶复合物被封装在阳离子交换的Y型沸石(M-Na-Y:M = K +,Cs +,Mg 2 +,Ca 2 +,NH 4 +,TMA +和TBA +)中,在三种类型的溶剂(CH 3 CN,H 2 O和CH 3 CN + H 2 O(1:1))中研究了用过氧化氢(H 2 O 2)将苯氧化为苯酚的催化活性。不论溶剂种类如何,[Fe(bpy)3 ] 2+中的抗衡阳离子M-Na-Y不影响对苯酚的选择性。CH 3 CN + H 2 O(1:1)中[Fe(bpy)3 ] 2+ @ M-Na-Y的催化活性没有显着差异,而每个CH 3 CN和H 2 O溶剂。有人认为,催化活性与苯在[Fe(bpy)3 ] 2+中的[Fe(bpy)3 ] 2+位点的可及性有关。@ M-Na-Y,由引入的抗衡阳离子的水合离子半径控制。通过比较在[Fe(bpy)3 ] 2+ @ Na-Y,[Fe(phen)3 ]上环烃(苯,环己烷和环己烯)的氧化催化活性,研究了配体与Fe离子配位的作用。2+ @ Na-Y和[Fe(terpy)2 ] 2+ @ Na-Y(phen = 1,10-菲咯啉,terpy = 2,2'; 6',2''-叔吡啶)。有人认为,π电子在诸如phen和terpy之类的配体上的扩展提高了具有π电子的底物如苯的吸收能力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号