当前位置:
X-MOL 学术
›
J. Membr. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Elucidating the mechanisms underlying the difference between chloride and nitrate rejection in nanofiltration
Journal of Membrane Science ( IF 9.5 ) Pub Date : 2018-02-01 , DOI: 10.1016/j.memsci.2017.10.049 Razi Epsztein , Wei Cheng , Evyatar Shaulsky , Nadir Dizge , Menachem Elimelech
Journal of Membrane Science ( IF 9.5 ) Pub Date : 2018-02-01 , DOI: 10.1016/j.memsci.2017.10.049 Razi Epsztein , Wei Cheng , Evyatar Shaulsky , Nadir Dizge , Menachem Elimelech
|
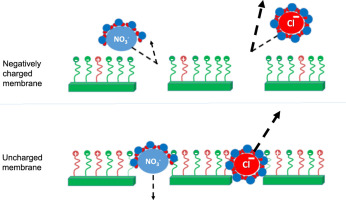
Abstract Despite the strong similarity between chloride (Cl-) and nitrate (NO3-) anions in terms of their hydrated radius and charge, Cl- is rejected more favorably than NO3- by nanofiltration (NF) membranes. The main goal of this study is to provide a better understanding of the removal mechanisms favoring the higher rejection of Cl- over NO3- in NF. A series of experiments with polyamide (NF270) and cellulose acetate (CK) NF membranes at different pH values, followed by calculation of the activation energies for Cl- and NO3- passage through the membranes, showed that the higher Cl- than NO3- rejection is attributed to both size-exclusion and Donnan (charge)-exclusion mechanisms. At a neutral membrane charge, a size-exclusion mechanism dominates the rejection of both anions. In this case, we observe higher rejection of Cl- over NO3- due to the lower hydration energy of NO3-, which corresponds to higher degree of dehydration and thus higher rate of passage through the NF membrane pores. At a negative membrane charge, the smaller volume of Cl- compared to NO3-, corresponding to higher ionic charge density, results in a stronger electrostatic repulsion of Cl- by the negatively charged membrane and therefore higher Cl- rejection than NO3-. The coupling of size- and Donnan-exclusion mechanisms with the NF270 membrane results in a maximum Cl- to NO3- rejection ratio at near the isoelectric pH where the membrane is slightly negatively charged. At a positive membrane charge, the sodium (Na+) counter ions dictate salt rejection independently of the anion type, resulting in almost similar rejections of Cl- and NO3-. Based on the insight gained from these experiments, a layer-by-layer (LbL) polyelectrolyte modification was applied to the NF270 membrane to control its surface charge. This modification showed that shifting the isoelectric point of the NF270 membrane from its original value (pH 4–5) to higher values (pH 6–9) increased the Cl- to NO3- rejection ratio at near neutral pH conditions, thus providing further support for our proposed mechanism underlying the difference between Cl- and NO3- rejection by NF membranes.
中文翻译:

阐明纳滤中氯化物和硝酸盐排斥之间差异的潜在机制
摘要 尽管氯离子 (Cl-) 和硝酸根 (NO3-) 阴离子在它们的水合半径和电荷方面具有很强的相似性,但通过纳滤 (NF) 膜,Cl- 比 NO3- 更容易被拒绝。本研究的主要目标是更好地了解有利于 NF 中 Cl- 的去除率高于 NO3- 的去除机制。使用聚酰胺 (NF270) 和醋酸纤维素 (CK) NF 膜在不同 pH 值下进行的一系列实验,然后计算 Cl- 和 NO3- 通过膜的活化能,表明 Cl- 比 NO3- 截留率更高归因于大小排除和唐南(电荷)排除机制。在中性膜电荷下,尺寸排阻机制支配着两种阴离子的排斥。在这种情况下,由于 NO3- 的较低水合能,我们观察到 Cl- 比 NO3- 更高的截留率,这对应于更高的脱水程度和更高的通过 NF 膜孔的速率。在负膜电荷下,与 NO3- 相比,Cl- 的体积较小,对应于更高的离子电荷密度,导致带负电荷的膜对 Cl- 更强的静电排斥,因此 Cl- 截留率高于 NO3-。NF270 膜将尺寸和唐南排除机制与 NF270 膜相结合,在接近等电 pH 值时产生最大的 Cl- 与 NO3- 截留率,此时膜带轻微负电荷。在膜正电荷时,钠 (Na+) 抗衡离子决定了独立于阴离子类型的盐截留率,导致 Cl- 和 NO3- 的截留率几乎相似。基于从这些实验中获得的见解,将逐层 (LbL) 聚电解质改性应用于 NF270 膜以控制其表面电荷。这种修改表明,将 NF270 膜的等电点从其原始值(pH 4-5)转移到更高的值(pH 6-9)在接近中性 pH 条件下增加了 Cl- 对 NO3- 的截留率,从而提供了进一步的支持对于我们提出的 NF 膜对 Cl- 和 NO3- 排斥之间差异的潜在机制。
更新日期:2018-02-01
中文翻译:

阐明纳滤中氯化物和硝酸盐排斥之间差异的潜在机制
摘要 尽管氯离子 (Cl-) 和硝酸根 (NO3-) 阴离子在它们的水合半径和电荷方面具有很强的相似性,但通过纳滤 (NF) 膜,Cl- 比 NO3- 更容易被拒绝。本研究的主要目标是更好地了解有利于 NF 中 Cl- 的去除率高于 NO3- 的去除机制。使用聚酰胺 (NF270) 和醋酸纤维素 (CK) NF 膜在不同 pH 值下进行的一系列实验,然后计算 Cl- 和 NO3- 通过膜的活化能,表明 Cl- 比 NO3- 截留率更高归因于大小排除和唐南(电荷)排除机制。在中性膜电荷下,尺寸排阻机制支配着两种阴离子的排斥。在这种情况下,由于 NO3- 的较低水合能,我们观察到 Cl- 比 NO3- 更高的截留率,这对应于更高的脱水程度和更高的通过 NF 膜孔的速率。在负膜电荷下,与 NO3- 相比,Cl- 的体积较小,对应于更高的离子电荷密度,导致带负电荷的膜对 Cl- 更强的静电排斥,因此 Cl- 截留率高于 NO3-。NF270 膜将尺寸和唐南排除机制与 NF270 膜相结合,在接近等电 pH 值时产生最大的 Cl- 与 NO3- 截留率,此时膜带轻微负电荷。在膜正电荷时,钠 (Na+) 抗衡离子决定了独立于阴离子类型的盐截留率,导致 Cl- 和 NO3- 的截留率几乎相似。基于从这些实验中获得的见解,将逐层 (LbL) 聚电解质改性应用于 NF270 膜以控制其表面电荷。这种修改表明,将 NF270 膜的等电点从其原始值(pH 4-5)转移到更高的值(pH 6-9)在接近中性 pH 条件下增加了 Cl- 对 NO3- 的截留率,从而提供了进一步的支持对于我们提出的 NF 膜对 Cl- 和 NO3- 排斥之间差异的潜在机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号