Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2017-10-31 , DOI: 10.1016/j.bmc.2017.10.041 Tarosh S. Patel , Jaimin D. Bhatt , Satish F. Vanparia , Urmila H. Patel , Ritu B. Dixit , Chaitanya J. Chudasama , Bhavesh D. Patel , Bharat C. Dixit
|
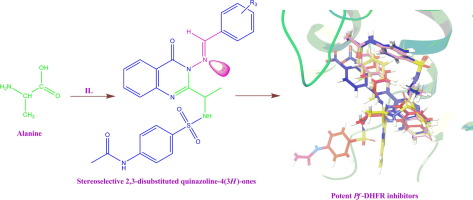
Grimmel’s method was optimized as well as modified leading to the cyclization and incorporation of alanine linked sulphonamide in 4-quinazolin-(3H)-ones. Further, the generation of heterocyclic motif at position-3 of 4-quinazolinones was explored by synthesis of imines, which unfortunately led to an isomeric mixture of stereoisomers. The hurdle of diastereomers encountered on the path was eminently rectified by development of new rapid and reproducible methodology involving the use of imidazolium based ionic liquid as solvents as well as catalyst for cyclization as well as synthesis of imines in situ at position-3 leading to procurement of single E-isomer as the target hybrid heterocyclic molecules. The purity and presence of single isomer was also confirmed by HPLC and spectroscopic techniques. Further, the synthesized sulphonamide linked 4-quinazolin-(3H)-ones hybrids were screened for their antimalarial potency rendering potent entities (4b, 4c, 4 l, 4 t and 4u). The active hybrids were progressively screened for enzyme inhibitory efficacy against presumed receptor Pf-DHFR and h-DHFR computationally as well as in vitro, proving their potency as dihydrofolate reductase inhibitors. The ADME properties of these active molecules were also predicted to enhance the knowhow of the oral bioavailability, indicating good bioavailability of the active entities.
中文翻译:

离子液体介导的丙氨酸连接杂化喹唑啉-4(3 H)-一衍生物的立体选择性合成,扰乱叶酸途径中的疟疾还原酶活性
对Grimmel的方法进行了优化和改进,从而使其在4-喹唑啉-(3 H)-环中环化并结合了丙氨酸连接的磺酰胺。此外,通过亚胺的合成探索了4-喹唑啉酮的位置3处的杂环基序的产生,不幸的是,这导致了立体异构体的异构体混合物。通过开发一种新的快速且可重现的方法,包括使用咪唑鎓离子液体作为溶剂以及环化催化剂以及在位置3上原位合成亚胺,导致采购,非常规方法的障碍已得到明显纠正。单个E的-异构体作为目标杂合杂环分子。单一异构体的纯度和存在也通过HPLC和光谱技术确认。此外,筛选了合成的磺酰胺连接的4-喹唑啉-(3 H)-ones杂种的抗疟疾效力增强实体(4b,4c,4l,4t和4u)。通过计算以及在体外逐步筛选活性杂种对假定的受体Pf -DHFR和h -DHFR的酶抑制作用,证明了其作为二氢叶酸还原酶抑制剂的功效。还预测了这些活性分子的ADME特性将增强口服生物利用度的知识,表明活性实体具有良好的生物利用度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号