当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An Evaluation of Multiple Catalytic Systems for the Cyanation of 2,3-Dichlorobenzoyl Chloride: Application to the Synthesis of Lamotrigine
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2017-10-31 00:00:00 , DOI: 10.1021/acs.oprd.7b00262 David C. Leitch 1 , Matthew P. John 2 , Paul A. Slavin 3 , Andrew D. Searle 3
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2017-10-31 00:00:00 , DOI: 10.1021/acs.oprd.7b00262 David C. Leitch 1 , Matthew P. John 2 , Paul A. Slavin 3 , Andrew D. Searle 3
Affiliation
|
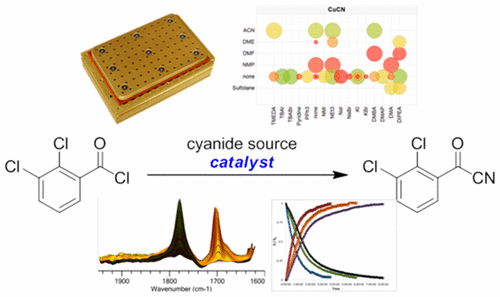
2,3-Dichlorobenzoyl cyanide is a key intermediate in the synthesis of Lamotrigine. An assessment of various catalytic systems for the cyanation of 2,3-dichlorobenzoyl chloride with cyanide salts is described. High-throughput experimentation identified many conditions for effecting the requisite chemistry, including amine bases and phase-transfer catalysts, as well as catalyst-free conditions utilizing acetonitrile as a polar cosolvent. A novel catalyst, CuBr2, was identified by consideration of the possible oxidation of Cu(I) during high-throughput screening experimentation. CuCN was found to be the best cyanide source for achieving clean conversion; however, the solubility of CuCN was the major factor limiting reaction rate under many conditions. Improving CuCN solubility by using acetonitrile as solvent enhanced the reaction rate even in the absence of the catalysts tested but significantly complicated isolation of the product. With no acetonitrile cosolvent, phase-transfer catalysts such as tetrabutylammonium bromide (TBABr) are effective; however, use of TBABr led to inconsistent reaction profiles from run-to-run, due to an unexpected clumping of the CuCN solid. Switching to cetyltrimethylammonium bromide (CTAB) alleviated this clumping behavior, leading to consistent reactivity. This CTAB-catalyzed process was scaled up, giving 560 kg of 2,3-dichlorobenzoyl cyanide in 77% isolated yield.
中文翻译:

2,3-二氯苯甲酰氯氰化反应的多种催化体系评价:在拉莫三嗪的合成中的应用
2,3-二氯苯甲酰氰是合成拉莫三嗪的关键中间体。描述了各种催化体系用于氰化2,3-二氯苯甲酰氯氰化的评估。高通量实验确定了影响必要化学反应的许多条件,包括胺碱和相转移催化剂,以及使用乙腈作为极性助溶剂的无催化剂条件。新型催化剂CuBr 2通过在高通量筛选实验中考虑到Cu(I)的可能氧化来确定。发现CuCN是实现清洁转化的最佳氰化物来源。然而,在许多条件下,CuCN的溶解度是限制反应速率的主要因素。通过使用乙腈作为溶剂来提高CuCN的溶解度,即使在没有测试的催化剂的情况下,也可以提高反应速率,但是产物的分离非常复杂。在没有乙腈助溶剂的情况下,相转移催化剂(如四丁基溴化铵(TBABr))是有效的。但是,由于CuCN固体的意外团聚,TBABr的使用导致每次运行的反应曲线不一致。改用十六烷基三甲基溴化铵(CTAB)减轻了这种结块现象,从而导致了始终如一的反应性。
更新日期:2017-11-01
中文翻译:

2,3-二氯苯甲酰氯氰化反应的多种催化体系评价:在拉莫三嗪的合成中的应用
2,3-二氯苯甲酰氰是合成拉莫三嗪的关键中间体。描述了各种催化体系用于氰化2,3-二氯苯甲酰氯氰化的评估。高通量实验确定了影响必要化学反应的许多条件,包括胺碱和相转移催化剂,以及使用乙腈作为极性助溶剂的无催化剂条件。新型催化剂CuBr 2通过在高通量筛选实验中考虑到Cu(I)的可能氧化来确定。发现CuCN是实现清洁转化的最佳氰化物来源。然而,在许多条件下,CuCN的溶解度是限制反应速率的主要因素。通过使用乙腈作为溶剂来提高CuCN的溶解度,即使在没有测试的催化剂的情况下,也可以提高反应速率,但是产物的分离非常复杂。在没有乙腈助溶剂的情况下,相转移催化剂(如四丁基溴化铵(TBABr))是有效的。但是,由于CuCN固体的意外团聚,TBABr的使用导致每次运行的反应曲线不一致。改用十六烷基三甲基溴化铵(CTAB)减轻了这种结块现象,从而导致了始终如一的反应性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号