Polymer ( IF 4.6 ) Pub Date : 2017-11-01 , DOI: 10.1016/j.polymer.2017.10.063 Rafael L. Schoch , Gustav Emilsson , Andreas B. Dahlin , Roderick Y.H. Lim
|
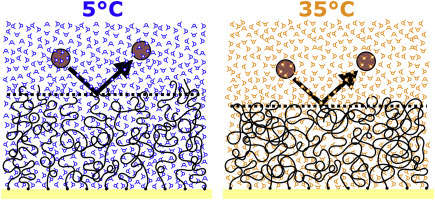
Poly(ethylene glycol) (PEG) is widely used in biotechnology-related applications yet its temperature-dependent functionality is not well understood. Here, we use bovine serum albumin (BSA) monomers and cross-linked dimers to directly probe the height of strongly stretched PEG brushes using surface plasmon resonance (SPR) in aqueous solution. Our results show that PEG brush height follows a smooth decrease as a function of increasing temperature commencing near room temperature. Measurements obtained by BSA monomers and dimers are comparable and suggest that BSA effectively probes the leading edge of the brush with minimal penetration into its interior being supported by SPR reflectivity calculations. Further, the BSA-PEG interaction remains largely inert over the entire temperature range. Overall, PEG brushes undergo a smooth conformational transition while fully preserving its protein excluding properties far from the lower critical solution temperature.
中文翻译:

蛋白质排阻通过温度敏感的PEG刷保存
聚(乙二醇)(PEG)广泛用于与生物技术有关的应用中,但其与温度有关的功能尚不为人所知。在这里,我们使用牛血清白蛋白(BSA)单体和交联的二聚体通过水溶液中的表面等离振子共振(SPR)直接探测强力拉伸的PEG刷的高度。我们的结果表明,PEG刷高度随着从室温附近开始的温度升高而平滑下降。由BSA单体和二聚体获得的测量结果具有可比性,并且表明BSA有效地探测了刷子的前缘,并通过SPR反射率计算支持了对刷子内部的最小渗透。此外,在整个温度范围内,BSA-PEG相互作用基本上保持惰性。全面的,



























 京公网安备 11010802027423号
京公网安备 11010802027423号