当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental and Computational Studies on Remote γ-C(sp3)–H Silylation and Germanylation of Aliphatic Carboxamides
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-11-01 00:00:00 , DOI: 10.1021/acscatal.7b03056 Arghya Deb 1 , Sukriti Singh 1 , Kapileswar Seth 1 , Sandeep Pimparkar 1 , Bangaru Bhaskararao 1 , Srimanta Guin 1 , Raghavan B. Sunoj 1 , Debabrata Maiti 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-11-01 00:00:00 , DOI: 10.1021/acscatal.7b03056 Arghya Deb 1 , Sukriti Singh 1 , Kapileswar Seth 1 , Sandeep Pimparkar 1 , Bangaru Bhaskararao 1 , Srimanta Guin 1 , Raghavan B. Sunoj 1 , Debabrata Maiti 1
Affiliation
|
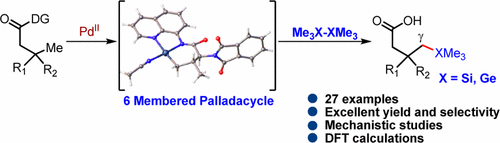
A Pd(II)-catalyzed protocol for highly regioselective distal γ-C–H silylation and germanylation of aliphatic carboxylic acids is reported. Bidentate 8-aminoquinoline as the directing group was found to stabilize the six-membered palladacycle. A variety of aliphatic carboxylic acids and amino acids were silylated and germanylated in good yields and high diasteroselectivities. Detailed mechanistic studies involving isolation of a Pd(II) intermediate, determination of the reaction rate and order, control experiments, and isotopic labeling and DFT studies were found to be crucial for elucidating the elementary steps involved in this distal aliphatic functionalization.
中文翻译:

脂肪族羧酰胺远程γ-C(sp 3)-H甲硅烷基化和德国化的实验和计算研究
据报道,Pd(II)催化的用于脂族羧酸的高度区域选择性的远端γ-C–H甲硅烷基化和锗烷基化的方案。二齿8-氨基喹啉是指导基团,可稳定六元的palladacycle。以高收率和高非对映选择性对各种脂族羧酸和氨基酸进行甲硅烷基化和锗基化。发现详细的机理研究涉及分离Pd(II)中间体,确定反应速率和顺序,进行对照实验以及同位素标记和DFT研究,对于阐明参与该远端脂肪族官能化的基本步骤至关重要。
更新日期:2017-11-01
中文翻译:

脂肪族羧酰胺远程γ-C(sp 3)-H甲硅烷基化和德国化的实验和计算研究
据报道,Pd(II)催化的用于脂族羧酸的高度区域选择性的远端γ-C–H甲硅烷基化和锗烷基化的方案。二齿8-氨基喹啉是指导基团,可稳定六元的palladacycle。以高收率和高非对映选择性对各种脂族羧酸和氨基酸进行甲硅烷基化和锗基化。发现详细的机理研究涉及分离Pd(II)中间体,确定反应速率和顺序,进行对照实验以及同位素标记和DFT研究,对于阐明参与该远端脂肪族官能化的基本步骤至关重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号