PLoS Pathogens ( IF 5.5 ) Pub Date : 2017-10-30 , DOI: 10.1371/journal.ppat.1006713 Kattareeya Kumthip 1, 2 , Darong Yang 1, 3 , Nan L Li 1 , Yunzhi Zhang 1, 4 , Meiyun Fan 5 , Aarti Sethuraman 5 , Kui Li 1
|
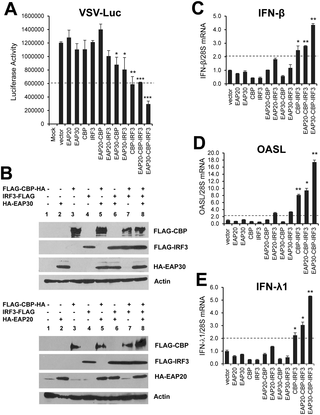
The activation of interferon (IFN)-regulatory factor-3 (IRF3), characterized by phosphorylation and nuclear translocation of the latent transcription factor, is central to initiating innate antiviral responses. Whereas much has been learned about the upstream pathways and signaling mechanisms leading to IRF3 activation, how activated IRF3 operates in the nucleus to control transcription of IFNs remains obscure. Here we identify EAP30 (a.k.a, SNF8/VPS22), an endosomal sorting complex required for transport (ESCRT)-II subunit, as an essential factor controlling IRF3-dependent antiviral defense. Depletion of EAP30, but not other ESCRT-II subunits, compromised IRF3-dependent induction of type I and III IFNs, IFN-stimulated genes (ISGs) and chemokines by double-stranded RNA or viruses. EAP30, however, was dispensable for the induction of inflammatory mediators of strict NF-κB target. Significantly, knockdown of EAP30 also impaired the establishment of an antiviral state against vesicular stomatitis virus and hepatitis C virus, which are of distinct viral families. Mechanistically, EAP30 was not required for IRF3 activation but rather acted at a downstream step. Specifically, a fraction of EAP30 localized within the nucleus, where it formed a complex with IRF3 and its transcriptional co-activator, CREB-binding protein (CBP), in a virus-inducible manner. These interactions promoted IRF3 binding to target gene promoters such as IFN-β, IFN-λ1 and ISG56. Together, our data describe an unappreciated role for EAP30 in IRF3-dependent innate antiviral response in the nucleus.
中文翻译:

ESCRT-II 复合物亚基 EAP30/SNF8 在 IRF3 依赖性先天抗病毒防御中的关键作用
干扰素 (IFN) 调节因子 3 (IRF3) 的激活以潜在转录因子的磷酸化和核转位为特征,对于启动先天抗病毒反应至关重要。尽管人们对导致 IRF3 激活的上游途径和信号传导机制已经了解很多,但激活的 IRF3 如何在细胞核中控制 IFN 的转录仍然不清楚。在这里,我们确定了 EAP30(又名 SNF8/VPS22),一种运输 (ESCRT)-II 亚基所需的内体分选复合物,作为控制 IRF3 依赖性抗病毒防御的重要因素。 EAP30(而非其他 ESCRT-II 亚基)的耗竭会损害双链 RNA 或病毒对 I 型和 III 型 IFN、IFN 刺激基因 (ISG) 和趋化因子的 IRF3 依赖性诱导。然而,EAP30 对于诱导严格 NF-κB 靶标的炎症介质来说是可有可无的。值得注意的是,EAP30 的敲低还损害了针对水泡性口炎病毒和丙型肝炎病毒的抗病毒状态的建立,这两种病毒属于不同的病毒家族。从机制上讲,EAP30 不是 IRF3 激活所必需的,而是在下游步骤中发挥作用。具体来说,EAP30 的一部分位于细胞核内,以病毒诱导的方式与 IRF3 及其转录共激活剂 CREB 结合蛋白 (CBP) 形成复合物。这些相互作用促进 IRF3 与靶基因启动子(如 IFN-β、IFN-λ1 和 ISG56)结合。总之,我们的数据描述了 EAP30 在细胞核内 IRF3 依赖性先天抗病毒反应中的未被认识到的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号