当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Applications of Photogating and Time Resolved Spectroscopy to Mechanistic Studies of Hydrogenases
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2017-10-30 00:00:00 , DOI: 10.1021/acs.accounts.7b00356 Brandon L. Greene 1 , Gregory E. Vansuch 2 , Bryant C. Chica 2 , Michael W. W. Adams 3 , R. Brian Dyer 2
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2017-10-30 00:00:00 , DOI: 10.1021/acs.accounts.7b00356 Brandon L. Greene 1 , Gregory E. Vansuch 2 , Bryant C. Chica 2 , Michael W. W. Adams 3 , R. Brian Dyer 2
Affiliation
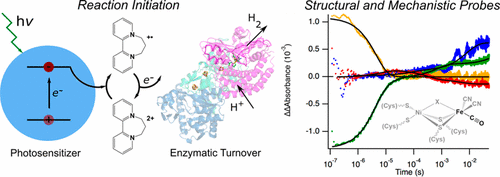
|
Rapid and facile redox chemistry is exemplified in nature by the oxidoreductases, the class of enzymes that catalyze electron transfer (ET) from a donor to an acceptor. The key role of oxidoreductases in metabolism and biosynthesis has imposed evolutionary pressure to enhance enzyme efficiency, pushing some toward the diffusion limit. Understanding the detailed molecular mechanisms of these highly optimized enzymes would provide an important foundation for the rational design of catalysts for multielectron chemistry, including fuel production. The hydrogenases (H2ases) are the oxidoreductases that catalyze the most basic electron and proton transfer reactions relevant to fuel production, the interconversion of protons and hydrogen, with kcat > 103 s–1. Thus, they provide a model system for studying the efficiency exhibited by oxidoreductases. Because of the extraordinarily fast catalytic rates of these enzymes, their mechanisms have been difficult to study directly but instead have been inferred from structural and steady-state measurements. Although informative, the kinetic competency of observed equilibrium steps can only be suggested by these methods, not demonstrated, because the fundamental (fast) catalytic steps remain unresolved, resulting in minimal insight regarding the underlying ET and proton transfer (PT) events.
中文翻译:

光选和时间分辨光谱在氢化酶机理研究中的应用
快速和简便的氧化还原化学反应本质上是氧化还原酶的例子,氧化还原酶是催化从供体到受体的电子转移(ET)的一类酶。氧化还原酶在代谢和生物合成中的关键作用施加了进化压力,以提高酶的效率,从而将某些酶推向扩散极限。了解这些高度优化的酶的详细分子机理将为合理设计多电子化学催化剂(包括燃料生产)提供重要的基础。氢化酶(H 2 ases)是氧化还原酶,可催化与燃料生产,质子和氢的相互转化有关的最基本的电子和质子转移反应,k cat > 10 3s –1。因此,他们提供了用于研究氧化还原酶效率的模型系统。由于这些酶的催化速率极快,因此很难直接研究其机理,而是从结构和稳态测量中推论得出。尽管提供了信息,但观察到的平衡步骤的动力学能力只能由这些方法提出,而没有得到证明,因为基本的(快速)催化步骤仍未解决,导致对潜在的ET和质子转移(PT)事件的了解最少。
更新日期:2017-10-30
中文翻译:

光选和时间分辨光谱在氢化酶机理研究中的应用
快速和简便的氧化还原化学反应本质上是氧化还原酶的例子,氧化还原酶是催化从供体到受体的电子转移(ET)的一类酶。氧化还原酶在代谢和生物合成中的关键作用施加了进化压力,以提高酶的效率,从而将某些酶推向扩散极限。了解这些高度优化的酶的详细分子机理将为合理设计多电子化学催化剂(包括燃料生产)提供重要的基础。氢化酶(H 2 ases)是氧化还原酶,可催化与燃料生产,质子和氢的相互转化有关的最基本的电子和质子转移反应,k cat > 10 3s –1。因此,他们提供了用于研究氧化还原酶效率的模型系统。由于这些酶的催化速率极快,因此很难直接研究其机理,而是从结构和稳态测量中推论得出。尽管提供了信息,但观察到的平衡步骤的动力学能力只能由这些方法提出,而没有得到证明,因为基本的(快速)催化步骤仍未解决,导致对潜在的ET和质子转移(PT)事件的了解最少。



























 京公网安备 11010802027423号
京公网安备 11010802027423号