当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Manganese‐Dioxide‐Catalyzed Trifluoromethylation and Azidation of Styrenyl Olefins via Radical Intermediates
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-11-14 , DOI: 10.1002/ajoc.201700508 Prabhakar Panday 1 , Parul Garg 1 , Anand Singh 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-11-14 , DOI: 10.1002/ajoc.201700508 Prabhakar Panday 1 , Parul Garg 1 , Anand Singh 1
Affiliation
|
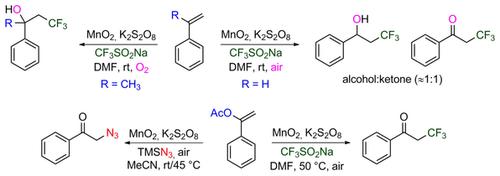
A manganese‐dioxide‐catalyzed trifluoromethylation of styrenes and enol acetates using the Langlois reagent is reported. Employing oxygen/air as the oxidant, styrenes afforded a mixture of the trifluoromethylacohol and ketone products in good overall yields. The protocol also works successfully for converting α‐methylstyrene derivatives to the corresponding trifluoromethyl alcohols. The keto‐trifluoromethylated products could be obtained exclusively when using enol acetates as substrates under the same reaction conditions. The method was extended to the radical azidation of enol acetates using trimethylsilyl azide to furnish the corresponding azido‐ketones in good yields.
中文翻译:

二氧化锰通过自由基中间体催化苯乙烯基烯烃的三氟甲基化和叠氮化
据报道,使用Langlois试剂可对苯乙烯和烯醇乙酸酯进行二氧化锰催化的三氟甲基化。利用氧气/空气作为氧化剂,苯乙烯以良好的总收率提供了三氟甲基醇和酮产物的混合物。该协议还可以成功地将α-甲基苯乙烯衍生物转化为相应的三氟甲基醇。当在相同反应条件下使用烯醇乙酸酯作为底物时,只能获得酮基三氟甲基化产物。该方法扩展到使用三甲基硅烷基叠氮化物进行乙酸烯醇乙酸酯的自由基叠氮化,从而以良好的产率提供相应的叠氮基酮。
更新日期:2017-11-14
中文翻译:

二氧化锰通过自由基中间体催化苯乙烯基烯烃的三氟甲基化和叠氮化
据报道,使用Langlois试剂可对苯乙烯和烯醇乙酸酯进行二氧化锰催化的三氟甲基化。利用氧气/空气作为氧化剂,苯乙烯以良好的总收率提供了三氟甲基醇和酮产物的混合物。该协议还可以成功地将α-甲基苯乙烯衍生物转化为相应的三氟甲基醇。当在相同反应条件下使用烯醇乙酸酯作为底物时,只能获得酮基三氟甲基化产物。该方法扩展到使用三甲基硅烷基叠氮化物进行乙酸烯醇乙酸酯的自由基叠氮化,从而以良好的产率提供相应的叠氮基酮。











































 京公网安备 11010802027423号
京公网安备 11010802027423号