当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of 2‐Cyclohexenone‐2‐carboxylate and 4‐Chloro‐2‐cyclohexenone‐2‐carboxylate Derivatives by Cyclization of Alkyne‐Tethered 1,3‐Ketoesters
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2017-11-29 , DOI: 10.1002/ajoc.201700510 Wilailak Kaewsri 1 , Krissada Norseeda 1 , Sureeporn Ruengsangtongkul 2 , Nattawadee Chaisan 1 , Charnsak Thongsornkleeb 1, 3 , Jumreang Tummatorn 1, 2 , Somsak Ruchirawat 1, 2
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2017-11-29 , DOI: 10.1002/ajoc.201700510 Wilailak Kaewsri 1 , Krissada Norseeda 1 , Sureeporn Ruengsangtongkul 2 , Nattawadee Chaisan 1 , Charnsak Thongsornkleeb 1, 3 , Jumreang Tummatorn 1, 2 , Somsak Ruchirawat 1, 2
Affiliation
|
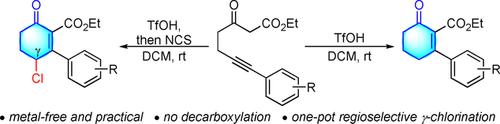
A method for the TfOH‐promoted (Tf=trifluoromethanesulfonyl) cyclization of alkyne‐tethered 1,3‐ketoesters to afford 2‐cyclohexenone‐2‐carboxylate derivatives has been developed. The procedure requires no transition‐metal reagent or catalyst and is practical to conduct under mild conditions. 2‐Cyclohexenone‐2‐carboxylate derivatives were obtained in moderate to good yields without any evidence of decarboxylation. In addition, the approach can be easily adapted as a one‐pot sequential cyclization/chlorination protocol that selectively provides access to 4‐chloro‐2‐cyclohexenone‐2‐carboxylate derivatives in moderate to good yields.
中文翻译:

炔烃系链的1,3-酮酸酯的环化反应合成2-环己烯酮-2-羧酸酯和4-氯-2-环己烯酮-2-羧酸酯衍生物
已经开发了一种方法,用于将TfOH促进的(Tf =三氟甲磺酰基)环烷基化的1,3-酮酸酯酯化,得到2-环己烯酮-2-羧酸酯衍生物。该程序不需要过渡金属试剂或催化剂,并且在温和条件下实际可行。2-环己烯酮-2-羧酸酯衍生物以中等至良好的收率获得,没有任何脱羧的迹象。此外,该方法可以轻松地用作单罐顺序环化/氯化方案,该方案可选择性地以中等到良好的产率提供4-氯-2-环己烯酮-2-羧酸酯衍生物的使用。
更新日期:2017-11-29
中文翻译:

炔烃系链的1,3-酮酸酯的环化反应合成2-环己烯酮-2-羧酸酯和4-氯-2-环己烯酮-2-羧酸酯衍生物
已经开发了一种方法,用于将TfOH促进的(Tf =三氟甲磺酰基)环烷基化的1,3-酮酸酯酯化,得到2-环己烯酮-2-羧酸酯衍生物。该程序不需要过渡金属试剂或催化剂,并且在温和条件下实际可行。2-环己烯酮-2-羧酸酯衍生物以中等至良好的收率获得,没有任何脱羧的迹象。此外,该方法可以轻松地用作单罐顺序环化/氯化方案,该方案可选择性地以中等到良好的产率提供4-氯-2-环己烯酮-2-羧酸酯衍生物的使用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号