当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparison of Various Means of Evaluating Molecular Electrostatic Potentials for Noncovalent Interactions
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2017-10-30 , DOI: 10.1002/jcc.25085 Steve Scheiner 1
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2017-10-30 , DOI: 10.1002/jcc.25085 Steve Scheiner 1
Affiliation
|
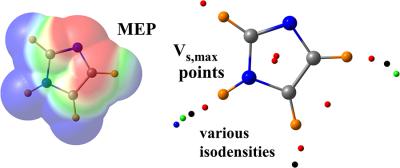
The various heterodimers formed by a series of Lewis acids with NH3 as Lewis base are identified. Lewis acids include those that can form chalcogen (HSF and HSBr), pnicogen (H2PF and H2PBr), and tetrel (H3SiF and H3SiBr) bonds, as well as H‐bonds and halogen bonds. The molecular electrostatic potential (MEP) of each Lewis acid is considered in a number of ways. Pictorial versions show broad regions of positive and negative MEP, on surfaces that vary with respect to either the value of the chosen isopotential, or their distance from the nuclei. Specific points are identified where the MEP reaches a maximum on a particular isodensity surface (Vs,max). The locations and values of Vs,max were evaluated on different isodensity surfaces, and compared to the stabilities of the various equilibrium geometries. As the chosen isodensity is decreased, and the MEP maxima drift away from the molecule, some points maintain their angular positions with respect to the molecule, whereas others undergo a reorientation. The lowering isodensity also causes some of the maxima to disappear. In general, there is a fairly good correlation between the energetic ordering of the equilibrium structures and the values of Vs,max. A number of possible Lewis acid sites on the heteroaromatic imidazole ring were also considered and presents some cautions about application of Vs,max as the principal criterion for predicting equilibrium geometries. © 2017 Wiley Periodicals, Inc.
中文翻译:

评估非共价相互作用的分子静电势的各种方法的比较
鉴定了由一系列以 NH3 作为路易斯碱的路易斯酸形成的各种异二聚体。路易斯酸包括那些可以形成硫属元素(HSF 和 HSBr)、pnicogen(H2PF 和 H2PBr)和四氢呋喃(H3SiF 和 H3SiBr)键,以及 H 键和卤素键的酸。以多种方式考虑每种路易斯酸的分子静电势 (MEP)。图形版本显示了正负 MEP 的广泛区域,在表面上随所选等电位值或它们与原子核的距离而变化。确定 MEP 在特定等密度表面 (Vs,max) 上达到最大值的特定点。Vs,max 的位置和值在不同的等密度表面上进行评估,并与各种平衡几何形状的稳定性进行比较。随着所选择的等密度降低,并且 MEP 最大值远离分子,一些点保持它们相对于分子的角位置,而其他点则经历重新定向。降低等密度也导致一些最大值消失。通常,平衡结构的能量排序与 Vs,max 值之间存在相当好的相关性。还考虑了杂芳族咪唑环上的许多可能的路易斯酸位点,并对应用 Vs,max 作为预测平衡几何形状的主要标准提出了一些警告。© 2017 威利期刊公司。平衡结构的能量排序与 Vs,max 值之间存在相当好的相关性。还考虑了杂芳族咪唑环上的许多可能的路易斯酸位点,并对应用 Vs,max 作为预测平衡几何形状的主要标准提出了一些警告。© 2017 威利期刊公司。平衡结构的能量排序与 Vs,max 值之间存在相当好的相关性。还考虑了杂芳族咪唑环上的许多可能的路易斯酸位点,并对应用 Vs,max 作为预测平衡几何形状的主要标准提出了一些警告。© 2017 威利期刊公司。
更新日期:2017-10-30
中文翻译:

评估非共价相互作用的分子静电势的各种方法的比较
鉴定了由一系列以 NH3 作为路易斯碱的路易斯酸形成的各种异二聚体。路易斯酸包括那些可以形成硫属元素(HSF 和 HSBr)、pnicogen(H2PF 和 H2PBr)和四氢呋喃(H3SiF 和 H3SiBr)键,以及 H 键和卤素键的酸。以多种方式考虑每种路易斯酸的分子静电势 (MEP)。图形版本显示了正负 MEP 的广泛区域,在表面上随所选等电位值或它们与原子核的距离而变化。确定 MEP 在特定等密度表面 (Vs,max) 上达到最大值的特定点。Vs,max 的位置和值在不同的等密度表面上进行评估,并与各种平衡几何形状的稳定性进行比较。随着所选择的等密度降低,并且 MEP 最大值远离分子,一些点保持它们相对于分子的角位置,而其他点则经历重新定向。降低等密度也导致一些最大值消失。通常,平衡结构的能量排序与 Vs,max 值之间存在相当好的相关性。还考虑了杂芳族咪唑环上的许多可能的路易斯酸位点,并对应用 Vs,max 作为预测平衡几何形状的主要标准提出了一些警告。© 2017 威利期刊公司。平衡结构的能量排序与 Vs,max 值之间存在相当好的相关性。还考虑了杂芳族咪唑环上的许多可能的路易斯酸位点,并对应用 Vs,max 作为预测平衡几何形状的主要标准提出了一些警告。© 2017 威利期刊公司。平衡结构的能量排序与 Vs,max 值之间存在相当好的相关性。还考虑了杂芳族咪唑环上的许多可能的路易斯酸位点,并对应用 Vs,max 作为预测平衡几何形状的主要标准提出了一些警告。© 2017 威利期刊公司。











































 京公网安备 11010802027423号
京公网安备 11010802027423号