当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A new approach to thiazoloisoindigo and derivatives using a lithium tetramethylpiperidine promoted cyclization to thiazoloisatin
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-10-23 00:00:00 , DOI: 10.1039/c7qo00841d Chenchen Li 1, 2, 3, 4, 5 , Huanrui Zhang 1, 2, 3, 4, 5 , Samuel Mirie 1, 2, 3, 4, 5 , Jiawei Peng 1, 2, 3, 4, 5 , Mian Cai 1, 2, 3, 4, 5 , Xiao Wang 1, 2, 3, 4, 5 , Zhenggang Lan 1, 2, 3, 4, 5 , Xiaobo Wan 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-10-23 00:00:00 , DOI: 10.1039/c7qo00841d Chenchen Li 1, 2, 3, 4, 5 , Huanrui Zhang 1, 2, 3, 4, 5 , Samuel Mirie 1, 2, 3, 4, 5 , Jiawei Peng 1, 2, 3, 4, 5 , Mian Cai 1, 2, 3, 4, 5 , Xiao Wang 1, 2, 3, 4, 5 , Zhenggang Lan 1, 2, 3, 4, 5 , Xiaobo Wan 1, 2, 3, 4, 5
Affiliation
|
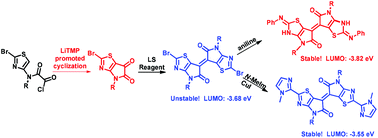
Brominated thiazoloisoindigo was synthesized for the first time from thiazoloisatin, which was obtained via an unprecedented nucleophilic cyclization strategy. Thiazoloisoindigo was shown to exhibit a deeper LUMO energy level than isoindigo and thienoisoindigo, which makes it a potential building block for n-type semiconductors. Moreover, brominated thiazoloisoindigo is sensitive toward nucleophilic attack, and its reaction with aniline resulted in an unexpectedly much more stable acceptor with a LUMO energy level as low as −3.82 eV.
中文翻译:

一种使用四甲基哌啶锂的噻唑异靛蓝及其衍生物的新方法可促进环化为噻唑异他汀
溴化噻唑异靛蓝是首次从噻唑异黄素合成的,噻唑异黄素是通过前所未有的亲核环化策略获得的。噻唑异靛蓝显示出比异靛蓝和噻吩并异靛蓝更深的LUMO能级,这使其成为n型半导体的潜在组成部分。此外,溴化噻唑异靛蓝对亲核攻击敏感,并且其与苯胺的反应产生了出乎意料的稳定得多的受体,其LUMO能级低至-3.82 eV。
更新日期:2017-10-30
中文翻译:

一种使用四甲基哌啶锂的噻唑异靛蓝及其衍生物的新方法可促进环化为噻唑异他汀
溴化噻唑异靛蓝是首次从噻唑异黄素合成的,噻唑异黄素是通过前所未有的亲核环化策略获得的。噻唑异靛蓝显示出比异靛蓝和噻吩并异靛蓝更深的LUMO能级,这使其成为n型半导体的潜在组成部分。此外,溴化噻唑异靛蓝对亲核攻击敏感,并且其与苯胺的反应产生了出乎意料的稳定得多的受体,其LUMO能级低至-3.82 eV。











































 京公网安备 11010802027423号
京公网安备 11010802027423号