当前位置:
X-MOL 学术
›
Faraday Discuss.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Optical backbone-sidechain charge transfer transitions in proteins sensitive to secondary structure and modifications
Faraday Discussions ( IF 3.3 ) Pub Date : 2017-10-30 , DOI: 10.1039/c7fd00203c I. Mandal 1, 2, 3, 4 , S. Paul 1, 2, 3, 4 , R. Venkatramani 1, 2, 3, 4
Faraday Discussions ( IF 3.3 ) Pub Date : 2017-10-30 , DOI: 10.1039/c7fd00203c I. Mandal 1, 2, 3, 4 , S. Paul 1, 2, 3, 4 , R. Venkatramani 1, 2, 3, 4
Affiliation
|
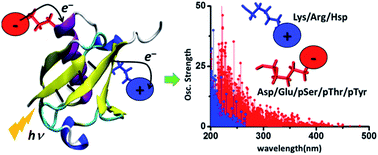
The absorption of light by proteins can induce charge transfer (CT) transitions in the UV-visible range of the electromagnetic spectrum. Metal–ligand complexes or active site prosthetic groups which absorb in the visible region exhibit prominent CT transitions. Furthermore, the protein backbone also exhibits CT transitions in the far UV range. In this manuscript, we present a detailed computational study of new near UV-visible CT transitions that involve amino acids with charged side chains. Specifically, using time dependent density functional theory calculations, we examine the absorption spectra of naturally charged amino acids (Lys, Glu, Arg, Asp and His), extracted from solution phase protein structures generated by classical molecular dynamics simulations, and phosphorylated amino acids (Tyr, Thr and Ser) from experimentally determined protein structures. We show that amino acids with charged sidechains present a directed electronic donor–bridge–acceptor paradigm, with the lowest energy optical excitations demonstrating peptide backbone-sidechain charge separations. The UV-visible spectral range of the backbone-sidechain CT transitions is determined by the chemical nature of the donor, bridge and acceptor groups within each amino acid, amino acid conformation and the protein secondary structure where the amino acids are located. Photoinduced CT occurs in opposite directions for the anionic and cationic amino acids along the ground state dipole moment vector for the chromophores. We find that photoinduced charge separation is more facile for the anionic amino acids (Asp, Glu, pSer, pThr and pTyr) relative to that for the cationic amino acids (Lys, Arg and Hsp). Our results provide a foundation for the development of spectroscopic markers based on the recently proposed Protein Charge Transfer Spectra (ProCharTS) which are relevant for the study of DNA-binding or intrinsically disordered proteins that are rich in charged amino acids.
中文翻译:

蛋白质的光学骨架侧链电荷转移转移对二级结构和修饰敏感
蛋白质对光的吸收可以在电磁波谱的紫外线可见范围内诱导电荷转移(CT)跃迁。在可见光区域吸收的金属-配体络合物或活性部位修复基团表现出明显的CT跃迁。此外,蛋白质主链在远紫外线范围内也表现出CT跃迁。在本手稿中,我们将对涉及具有带电荷侧链氨基酸的新型近紫外可见CT转换进行详细的计算研究。具体而言,使用随时间变化的密度泛函理论计算,我们检查了从经典分子动力学模拟生成的溶液相蛋白质结构中提取的天然带电氨基酸(Lys,Glu,Arg,Asp和His)的吸收光谱,以及磷酸化的氨基酸(提尔 Thr和Ser)来自实验确定的蛋白质结构。我们显示,带电荷侧链的氨基酸呈现出一种直接的电子供体-桥-受体范式,其最低能量的光激发表明了肽主链-侧链电荷的分离。骨架侧链CT跃迁的紫外可见光谱范围取决于每个氨基酸内的供体,桥和受体基团的化学性质,氨基酸构象和氨基酸所处的蛋白质二级结构。沿着生色基团的基态偶极矩向量,阴离子和阳离子氨基酸的光诱导CT发生在相反的方向。我们发现,对于阴离子氨基酸(Asp,Glu,pSer,pThr和pTyr)相对于阳离子氨基酸(Lys,Arg和Hsp)而言。我们的研究结果为基于最近提出的蛋白质电荷转移光谱(ProCharTS)的光谱标记物的开发提供了基础,该蛋白质标记物与富含电荷氨基酸的DNA结合或内在无序的蛋白质的研究有关。
更新日期:2018-04-17
中文翻译:

蛋白质的光学骨架侧链电荷转移转移对二级结构和修饰敏感
蛋白质对光的吸收可以在电磁波谱的紫外线可见范围内诱导电荷转移(CT)跃迁。在可见光区域吸收的金属-配体络合物或活性部位修复基团表现出明显的CT跃迁。此外,蛋白质主链在远紫外线范围内也表现出CT跃迁。在本手稿中,我们将对涉及具有带电荷侧链氨基酸的新型近紫外可见CT转换进行详细的计算研究。具体而言,使用随时间变化的密度泛函理论计算,我们检查了从经典分子动力学模拟生成的溶液相蛋白质结构中提取的天然带电氨基酸(Lys,Glu,Arg,Asp和His)的吸收光谱,以及磷酸化的氨基酸(提尔 Thr和Ser)来自实验确定的蛋白质结构。我们显示,带电荷侧链的氨基酸呈现出一种直接的电子供体-桥-受体范式,其最低能量的光激发表明了肽主链-侧链电荷的分离。骨架侧链CT跃迁的紫外可见光谱范围取决于每个氨基酸内的供体,桥和受体基团的化学性质,氨基酸构象和氨基酸所处的蛋白质二级结构。沿着生色基团的基态偶极矩向量,阴离子和阳离子氨基酸的光诱导CT发生在相反的方向。我们发现,对于阴离子氨基酸(Asp,Glu,pSer,pThr和pTyr)相对于阳离子氨基酸(Lys,Arg和Hsp)而言。我们的研究结果为基于最近提出的蛋白质电荷转移光谱(ProCharTS)的光谱标记物的开发提供了基础,该蛋白质标记物与富含电荷氨基酸的DNA结合或内在无序的蛋白质的研究有关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号