PLoS Pathogens ( IF 6.7 ) Pub Date : 2017-10-27 , DOI: 10.1371/journal.ppat.1006667 David Sychantha 1 , Carys S Jones 1 , Dustin J Little 2, 3 , Patrick J Moynihan 4 , Howard Robinson 5 , Nicola F Galley 6 , David I Roper 6 , Christopher G Dowson 6 , P Lynne Howell 2, 3 , Anthony J Clarke 1
|
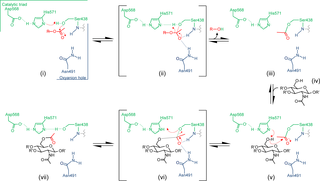
The O-acetylation of the essential cell wall polymer peptidoglycan occurs in most Gram-positive bacterial pathogens, including species of Staphylococcus, Streptococcus and Enterococcus. This modification to peptidoglycan protects these pathogens from the lytic action of the lysozymes of innate immunity systems and, as such, is recognized as a virulence factor. The key enzyme involved, peptidoglycan O-acetyltransferase A (OatA) represents a particular challenge to biochemical study since it is a membrane associated protein whose substrate is the insoluble peptidoglycan cell wall polymer. OatA is predicted to be bimodular, being comprised of an N-terminal integral membrane domain linked to a C-terminal extracytoplasmic domain. We present herein the first biochemical and kinetic characterization of the C-terminal catalytic domain of OatA from two important human pathogens, Staphylococcus aureus and Streptococcus pneumoniae. Using both pseudosubstrates and novel biosynthetically-prepared peptidoglycan polymers, we characterized distinct substrate specificities for the two enzymes. In addition, the high resolution crystal structure of the C-terminal domain reveals an SGNH/GDSL-like hydrolase fold with a catalytic triad of amino acids but with a non-canonical oxyanion hole structure. Site-specific replacements confirmed the identity of the catalytic and oxyanion hole residues. A model is presented for the O-acetylation of peptidoglycan whereby the translocation of acetyl groups from a cytoplasmic source across the cytoplasmic membrane is catalyzed by the N-terminal domain of OatA for their transfer to peptidoglycan by its C-terminal domain. This study on the structure-function relationship of OatA provides a molecular and mechanistic understanding of this bacterial resistance mechanism opening the prospect for novel chemotherapeutic exploration to enhance innate immunity protection against Gram-positive pathogens.
中文翻译:

革兰氏阳性病原体抗毒靶点的体外表征,肽聚糖 O-乙酰转移酶 A (OatA)
基本细胞壁聚合物肽聚糖的 O-乙酰化发生在大多数革兰氏阳性细菌病原体中,包括葡萄球菌、链球菌和肠球菌。这种对肽聚糖的修饰保护这些病原体免受先天免疫系统溶菌酶的溶解作用,因此被认为是一种毒力因子。涉及的关键酶,肽聚糖O-乙酰转移酶 A (OatA) 对生化研究提出了特殊挑战,因为它是一种膜相关蛋白,其底物是不溶性肽聚糖细胞壁聚合物。OatA 预计是双模的,由连接到 C 末端胞质外结构域的 N 末端完整膜结构域组成。我们在此首次展示了来自两种重要人类病原体、金黄色葡萄球菌和肺炎链球菌的 OatA C 末端催化结构域的生化和动力学特征。. 使用假底物和新型生物合成制备的肽聚糖聚合物,我们表征了两种酶的不同底物特异性。此外,C 端结构域的高分辨率晶体结构揭示了一个类似 SGNH/GDSL 的水解酶折叠,具有氨基酸的催化三联体,但具有非规范的氧阴离子空穴结构。位点特异性替换证实了催化和氧阴离子空穴残基的身份。提出了肽聚糖的 O-乙酰化模型,其中乙酰基从细胞质来源穿过细胞质膜的易位由 OatA 的 N 端结构域催化,通过其 C 端结构域转移到肽聚糖。



























 京公网安备 11010802027423号
京公网安备 11010802027423号