当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organocatalytic Enantioselective Friedel–Crafts Alkylation/Lactonization Reaction of Hydroxyindoles with Methyleneoxindoles
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-12-13 , DOI: 10.1002/adsc.201701089 Mengjie Xiao 1, 2 , Dengfeng Xu 1 , Weihong Liang 1, 2 , Wenyu Wu 2 , Albert S. C. Chan 3 , Junling Zhao 3
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-12-13 , DOI: 10.1002/adsc.201701089 Mengjie Xiao 1, 2 , Dengfeng Xu 1 , Weihong Liang 1, 2 , Wenyu Wu 2 , Albert S. C. Chan 3 , Junling Zhao 3
Affiliation
|
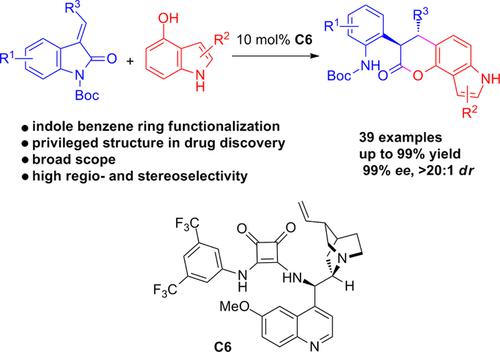
Functionalization of the indole benzene ring was achieved by using an organocatalytic enantioselective Friedel–Crafts alkylation/lactonization reaction of hydroxyindoles with a variety of substituted methyleneoxindoles. This reaction was applicable to indoles substituted with the hydroxy group at different positions of the benzene ring, and the corresponding pyrrolodihydrocoumarins were obtained in moderate to high yields (37–99%) with high stereoselectivities (up to 99% ee and >20:1 dr) in most cases. A scale‐up reaction and derivatization of the representative products were also carried out to investigate the usefulness of this protocol.
中文翻译:

羟基吲哚与亚甲基恶唑的有机催化对映选择性Friedel-Crafts烷基化/内酯化反应
吲哚苯环的官能化通过使用羟基吲哚与各种取代的亚甲基吲哚的有机催化对映选择性Friedel-Crafts烷基化/内酯化反应来实现。该反应适用于在苯环的不同位置被羟基取代的吲哚,并以中等到高产率(37-99%)和高立体选择性(高达99%ee和> 20:1 )获得相应的吡咯二氢香豆素博士)在大多数情况下。还进行了放大反应和代表产品的衍生化,以研究该方案的有用性。
更新日期:2017-12-13
中文翻译:

羟基吲哚与亚甲基恶唑的有机催化对映选择性Friedel-Crafts烷基化/内酯化反应
吲哚苯环的官能化通过使用羟基吲哚与各种取代的亚甲基吲哚的有机催化对映选择性Friedel-Crafts烷基化/内酯化反应来实现。该反应适用于在苯环的不同位置被羟基取代的吲哚,并以中等到高产率(37-99%)和高立体选择性(高达99%ee和> 20:1 )获得相应的吡咯二氢香豆素博士)在大多数情况下。还进行了放大反应和代表产品的衍生化,以研究该方案的有用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号