当前位置:
X-MOL 学术
›
Macromol. Rapid Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One‐Pot Synthesis of Thermoresponsive Amyloidogenic Peptide–Polymer Conjugates via Thio–Bromo “Click” Reaction of RAFT Polymers
Macromolecular Rapid Communications ( IF 4.2 ) Pub Date : 2017-10-27 , DOI: 10.1002/marc.201700507 Sonu Kumar 1 , Stefanie Deike 1 , Wolfgang H. Binder 1
Macromolecular Rapid Communications ( IF 4.2 ) Pub Date : 2017-10-27 , DOI: 10.1002/marc.201700507 Sonu Kumar 1 , Stefanie Deike 1 , Wolfgang H. Binder 1
Affiliation
|
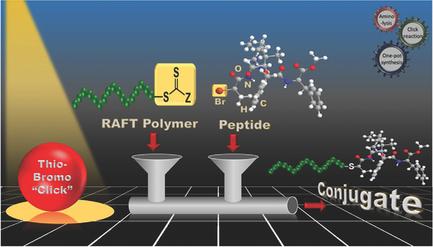
A synthetic strategy to efficiently prepare main‐chain peptide–polymer conjugates probing their aggregation in solution is described. An in situ tandem reaction based on aminolysis/thio–bromo “click” reaction is performed to tether an amyloidogenic peptide fragment amyloid‐β17–20 (Leu‐Val‐Phe‐Phe (LVFF)) to the ω‐chain end of poly(diethylene glycol methyl ether acrylate) (PDEGA), prepared via reversible addition fragmentation chain transfer polymerization. Structural confirmation of the constructed conjugates PDEGA–LVFF (Mn,SEC = 5600, Ð = 1.21), (Mn,SEC = 7600, Ð = 1.16), and (Mn,SEC = 8900, Ð = 1.15) is successfully made by combined studies of 1H NMR, size‐exclusion chromatography, matrix‐assisted laser desorption ionization time‐of‐flight (MALDI‐TOF) mass spectrometry, and electrospray ionization time‐of‐flight (ESI‐TOF) mass spectrometry. The effect of the peptidic constituent on the thermoresponsive behavior of the polymer is examined by UV–vis spectroscopy, and the self‐assembly behavior of the amphiphilic conjugate is further exploited, exhibiting micellar morphology in aqueous solution.
中文翻译:

通过RAFT聚合物的硫代-溴“点击”反应单反应合成热敏性淀粉样肽-聚合物
描述了一种有效制备探测其在溶液中聚集的有效方法的主链肽-聚合物共轭物的合成策略。一种基于氨解/硫代溴原位串联反应“点击”进行反应系绳淀粉样蛋白肽片段的淀粉样蛋白β 17-20(亮氨酸-VAL-PHE-PHE(LVFF)),以聚ω链末端(二甘醇甲基醚丙烯酸酯)(PDEGA),是通过可逆的加成断裂链转移聚合制备的。构造的共轭物PDEGA-LVFF的结构确认(M n,SEC = 5600,Ð = 1.21),(M n,SEC = 7600,Ð = 1.16),和(M n,SEC = 8900,Ð= 1.15)是通过1 H NMR,尺寸排阻色谱,基质辅助激光解吸电离飞行时间(MALDI-TOF)质谱和电喷雾电离飞行时间(ESI-TOF )的组合研究成功完成的) 质谱。紫外可见光谱检查了肽类成分对聚合物热响应行为的影响,并进一步利用了两亲性结合物的自组装行为,在水溶液中表现出胶束形态。
更新日期:2017-10-27
中文翻译:

通过RAFT聚合物的硫代-溴“点击”反应单反应合成热敏性淀粉样肽-聚合物
描述了一种有效制备探测其在溶液中聚集的有效方法的主链肽-聚合物共轭物的合成策略。一种基于氨解/硫代溴原位串联反应“点击”进行反应系绳淀粉样蛋白肽片段的淀粉样蛋白β 17-20(亮氨酸-VAL-PHE-PHE(LVFF)),以聚ω链末端(二甘醇甲基醚丙烯酸酯)(PDEGA),是通过可逆的加成断裂链转移聚合制备的。构造的共轭物PDEGA-LVFF的结构确认(M n,SEC = 5600,Ð = 1.21),(M n,SEC = 7600,Ð = 1.16),和(M n,SEC = 8900,Ð= 1.15)是通过1 H NMR,尺寸排阻色谱,基质辅助激光解吸电离飞行时间(MALDI-TOF)质谱和电喷雾电离飞行时间(ESI-TOF )的组合研究成功完成的) 质谱。紫外可见光谱检查了肽类成分对聚合物热响应行为的影响,并进一步利用了两亲性结合物的自组装行为,在水溶液中表现出胶束形态。











































 京公网安备 11010802027423号
京公网安备 11010802027423号