当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Energetic analysis of conjugated hydrocarbons using the interacting quantum atoms method
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2017-10-26 , DOI: 10.1002/jcc.25089 Jesús Jara-Cortés 1 , Jesús Hernández-Trujillo 1
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2017-10-26 , DOI: 10.1002/jcc.25089 Jesús Jara-Cortés 1 , Jesús Hernández-Trujillo 1
Affiliation
|
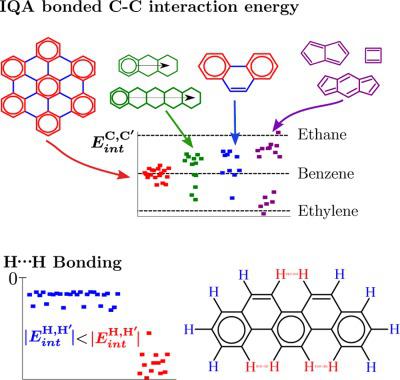
A number of aromatic, antiaromatic, and nonaromatic organic molecules was analyzed in terms of the contributions to the electronic energy defined in the quantum theory of atoms in molecules and the interacting quantum atoms method. Regularities were found in the exchange and electrostatic interatomic energies showing trends that are closely related to those of the delocalization indices defined in the theory. In particular, the CC interaction energies between bonded atoms allow to rationalize the energetic stabilization associated with the bond length alternation in conjugated polyenes. This approach also provides support to Clar's sextet rules devised for aromatic systems. In addition, the H ⋯ H bonding found in some of the aromatic molecules studied was of an attractive nature, according to the stabilizing exchange interaction between the bonded H atoms. © 2017 Wiley Periodicals, Inc.
中文翻译:

使用相互作用的量子原子方法对共轭烃进行能量分析
根据分子中原子的量子理论和相互作用的量子原子方法中定义的电子能量的贡献,分析了许多芳香族、反芳香族和非芳香族有机分子。在交换和静电原子间能量中发现了规律,显示出与理论中定义的离域指数密切相关的趋势。特别是,键合原子之间的 CC 相互作用能使得与共轭多烯中键长交替相关的能量稳定性合理化。这种方法还支持 Clar 为芳香系统设计的六重奏规则。此外,在研究的一些芳香分子中发现的 H⋯H 键具有吸引力,根据键合 H 原子之间的稳定交换相互作用。© 2017 威利期刊公司。
更新日期:2017-10-26
中文翻译:

使用相互作用的量子原子方法对共轭烃进行能量分析
根据分子中原子的量子理论和相互作用的量子原子方法中定义的电子能量的贡献,分析了许多芳香族、反芳香族和非芳香族有机分子。在交换和静电原子间能量中发现了规律,显示出与理论中定义的离域指数密切相关的趋势。特别是,键合原子之间的 CC 相互作用能使得与共轭多烯中键长交替相关的能量稳定性合理化。这种方法还支持 Clar 为芳香系统设计的六重奏规则。此外,在研究的一些芳香分子中发现的 H⋯H 键具有吸引力,根据键合 H 原子之间的稳定交换相互作用。© 2017 威利期刊公司。











































 京公网安备 11010802027423号
京公网安备 11010802027423号