当前位置:
X-MOL 学术
›
Adv. Healthcare Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rational Design of a New Self‐Codelivery System from Redox‐Sensitive Camptothecin–Cytarabine Conjugate Assembly for Effectively Synergistic Anticancer Therapy
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2017-10-27 , DOI: 10.1002/adhm.201700829 Wenxiu He 1 , Xu Hu 1 , Wei Jiang 1 , Ruiling Liu 1 , Di Zhang 1 , Jing Zhang 1 , Zhonghao Li 2 , Yuxia Luan 1
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2017-10-27 , DOI: 10.1002/adhm.201700829 Wenxiu He 1 , Xu Hu 1 , Wei Jiang 1 , Ruiling Liu 1 , Di Zhang 1 , Jing Zhang 1 , Zhonghao Li 2 , Yuxia Luan 1
Affiliation
|
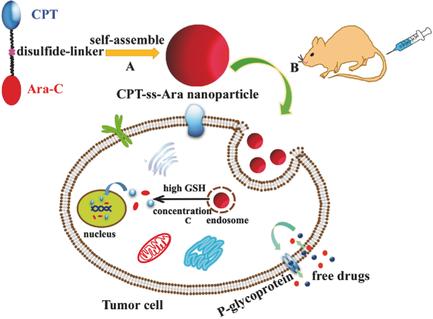
Herein, two careful selected anticancer drugs camptothecin (CPT) and cytarabine (Ara‐C) with different biological action mechanisms and different water solubility are conjugated together through a glutathione (GSH) cleavable disulfide bond to construct a redox‐sensitive drug–drug conjugate, which can self‐assemble into nanoparticles, thus notably improving the water solubility of CPT and the cell membrane permeability of Ara‐C. Compared with free drugs, the self‐assembled CPT‐ss‐Ara nanoparticles can concentrate in tumor tissues through the enhanced permeability and retention (EPR) effect, then they can be rapidly internalized by tumor cells and degrade into free drugs for killing the tumor cells when exposed to the reductive environment (GSH) of tumor cells, thereby reducing the injury to normal cells. Meanwhile, the CPT‐ss‐Ara nanoparticles can effectively protect CPT and Ara‐C molecules from biological inactivation before their arrival in tumor microenvironment since free CPT and Ara‐C are easy to partly lose their therapy efficacy due to their structure degradation in blood circulation. The in vitro and in vivo anticancer experimental results indicate that simultaneous release of free CPT and Ara‐C can realize synergistic chemotherapy effects, thus markedly improve their anticancer activity. Therefore, our designed carrier‐free, redox‐sensitive CPT‐ss‐Ara nanoparticles might have promising clinical application to combat cancers.
中文翻译:

氧化还原敏感性喜树碱-阿糖胞苷偶联物组件的新型自我密码传递系统的合理设计,可有效进行协同抗癌治疗
在此,通过谷胱甘肽(GSH)可裂解的二硫键将两种经过仔细选择的抗癌药物喜树碱(CPT)和阿糖胞苷(Ara‐C)通过谷胱甘肽(GSH)可裂解的二硫键结合在一起,构建了对氧化还原敏感的药物-药物结合物,它可以自组装成纳米颗粒,从而显着提高了CPT的水溶性和Ara-C的细胞膜通透性。与游离药物相比,自组装的CPT-ss-Ara纳米颗粒可以通过增强的通透性和保留(EPR)效应集中在肿瘤组织中,然后可以被肿瘤细胞快速内化并降解为游离药物以杀死肿瘤细胞。当暴露于肿瘤细胞的还原性环境(GSH)时,从而减少了对正常细胞的伤害。同时,CPT-ss-Ara纳米粒子可以有效地保护CPT和Ara-C分子进入肿瘤微环境,使其免受生物学失活,因为游离CPT和Ara-C由于血液循环中的结构退化而易于部分失去治疗功效。体外和体内抗癌实验结果表明,游离CPT和Ara-C的同时释放可实现协同化疗作用,从而显着提高其抗癌活性。因此,我们设计的无载体,对氧化还原敏感的CPT-ss-Ara纳米颗粒可能具有抗癌的临床应用前景。
更新日期:2017-10-27
中文翻译:

氧化还原敏感性喜树碱-阿糖胞苷偶联物组件的新型自我密码传递系统的合理设计,可有效进行协同抗癌治疗
在此,通过谷胱甘肽(GSH)可裂解的二硫键将两种经过仔细选择的抗癌药物喜树碱(CPT)和阿糖胞苷(Ara‐C)通过谷胱甘肽(GSH)可裂解的二硫键结合在一起,构建了对氧化还原敏感的药物-药物结合物,它可以自组装成纳米颗粒,从而显着提高了CPT的水溶性和Ara-C的细胞膜通透性。与游离药物相比,自组装的CPT-ss-Ara纳米颗粒可以通过增强的通透性和保留(EPR)效应集中在肿瘤组织中,然后可以被肿瘤细胞快速内化并降解为游离药物以杀死肿瘤细胞。当暴露于肿瘤细胞的还原性环境(GSH)时,从而减少了对正常细胞的伤害。同时,CPT-ss-Ara纳米粒子可以有效地保护CPT和Ara-C分子进入肿瘤微环境,使其免受生物学失活,因为游离CPT和Ara-C由于血液循环中的结构退化而易于部分失去治疗功效。体外和体内抗癌实验结果表明,游离CPT和Ara-C的同时释放可实现协同化疗作用,从而显着提高其抗癌活性。因此,我们设计的无载体,对氧化还原敏感的CPT-ss-Ara纳米颗粒可能具有抗癌的临床应用前景。









































 京公网安备 11010802027423号
京公网安备 11010802027423号