当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isoform-Specific Substrate Inhibition Mechanism of Human Tryptophan Hydroxylase
Biochemistry ( IF 2.9 ) Pub Date : 2017-10-27 00:00:00 , DOI: 10.1021/acs.biochem.7b00763 Kasper D. Tidemand 1 , Günther H. Peters 1 , Pernille Harris 1 , Eva Stensgaard 1 , Hans E. M. Christensen 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-10-27 00:00:00 , DOI: 10.1021/acs.biochem.7b00763 Kasper D. Tidemand 1 , Günther H. Peters 1 , Pernille Harris 1 , Eva Stensgaard 1 , Hans E. M. Christensen 1
Affiliation
|
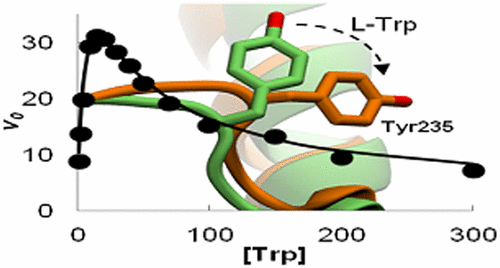
Tryptophan hydroxylase (TPH) catalyzes the initial and rate-limiting step in the biosynthesis of serotonin, which is associated with a variety of disorders such as depression and irritable bowel syndrome. TPH exists in two isoforms: TPH1 and TPH2. TPH1 catalyzes the initial step in the synthesis of serotonin in the peripheral tissues, while TPH2 catalyzes this step in the brain. In this study, the steady-state kinetic mechanism for the catalytic domain of human TPH1 has been determined. Varying substrate tryptophan (Trp) and tetrahydrobiopterin (BH4) results in a hybrid Ping Pong-ordered mechanism in which the reaction can either occur through a Ping Pong or a sequential mechanism depending on the concentration of tryptophan. The catalytic domain of TPH1 shares a sequence identity of 81% with TPH2. Despite the high sequence identity, differences in the kinetic parameters of the isoforms have been identified; i.e., only TPH1 displays substrate tryptophan inhibition. This study demonstrates that the difference can be traced to an active site loop which displays different properties in the TPH isoforms. Steady-state kinetic results of the isoforms, and variants with point mutations in a loop lining the active site, show that the kinetic parameters of only TPH1 are significantly changed upon mutations. Mutations in the active site loop of TPH1 result in an increase in the substrate inhibition constant, Ki, and therefore turnover rate. Molecular dynamics simulations reveal that this substrate inhibition mechanism occurs through a closure of the cosubstrate, BH4, binding pocket, which is induced by Trp binding.
中文翻译:

人类色氨酸羟化酶的异构体特异性底物抑制机理
色氨酸羟化酶(TPH)催化5-羟色胺生物合成的初始步骤和限速步骤,该步骤与多种疾病(例如抑郁症和肠易激综合症)有关。TPH存在两种同工型:TPH1和TPH2。TPH1催化周围组织中5-羟色胺合成的起始步骤,而TPH2则催化大脑中的该步骤。在这项研究中,已确定了人类TPH1催化域的稳态动力学机制。各种底物色氨酸(Trp)和四氢生物蝶呤(BH 4)导致混合乒乓有序机制,根据色氨酸的浓度,反应可以通过乒乓发生,也可以通过顺序机制发生。TPH1的催化结构域与TPH2的序列同一性为81%。尽管序列同一性很高,但已鉴定出同工型动力学参数的差异。即只有TPH1显示底物色氨酸抑制。这项研究表明,差异可以追溯到在TPH亚型中显示不同特性的活性位点环。同工型的稳态动力学结果以及在活性位点衬里的环中具有点突变的变体表明,只有TPH1的动力学参数会在突变后发生显着变化。K i,因此周转率。分子动力学模拟表明,这种底物抑制机制是通过共底物BH 4结合口袋的闭合而发生的,该结合底物是由Trp结合诱导的。
更新日期:2017-10-27
中文翻译:

人类色氨酸羟化酶的异构体特异性底物抑制机理
色氨酸羟化酶(TPH)催化5-羟色胺生物合成的初始步骤和限速步骤,该步骤与多种疾病(例如抑郁症和肠易激综合症)有关。TPH存在两种同工型:TPH1和TPH2。TPH1催化周围组织中5-羟色胺合成的起始步骤,而TPH2则催化大脑中的该步骤。在这项研究中,已确定了人类TPH1催化域的稳态动力学机制。各种底物色氨酸(Trp)和四氢生物蝶呤(BH 4)导致混合乒乓有序机制,根据色氨酸的浓度,反应可以通过乒乓发生,也可以通过顺序机制发生。TPH1的催化结构域与TPH2的序列同一性为81%。尽管序列同一性很高,但已鉴定出同工型动力学参数的差异。即只有TPH1显示底物色氨酸抑制。这项研究表明,差异可以追溯到在TPH亚型中显示不同特性的活性位点环。同工型的稳态动力学结果以及在活性位点衬里的环中具有点突变的变体表明,只有TPH1的动力学参数会在突变后发生显着变化。K i,因此周转率。分子动力学模拟表明,这种底物抑制机制是通过共底物BH 4结合口袋的闭合而发生的,该结合底物是由Trp结合诱导的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号