当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Consequences of Depsipeptide Substitution on the ClpP Activation Activity of Antibacterial Acyldepsipeptides
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2017-10-27 00:00:00 , DOI: 10.1021/acsmedchemlett.7b00320 Yangxiong Li , Nathan P. Lavey , Jesse A. Coker , Jessica E. Knobbe , Dat C. Truong , Hongtao Yu 1 , Yu-Shan Lin 1 , Susan L. Nimmo , Adam S. Duerfeldt
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2017-10-27 00:00:00 , DOI: 10.1021/acsmedchemlett.7b00320 Yangxiong Li , Nathan P. Lavey , Jesse A. Coker , Jessica E. Knobbe , Dat C. Truong , Hongtao Yu 1 , Yu-Shan Lin 1 , Susan L. Nimmo , Adam S. Duerfeldt
Affiliation
|
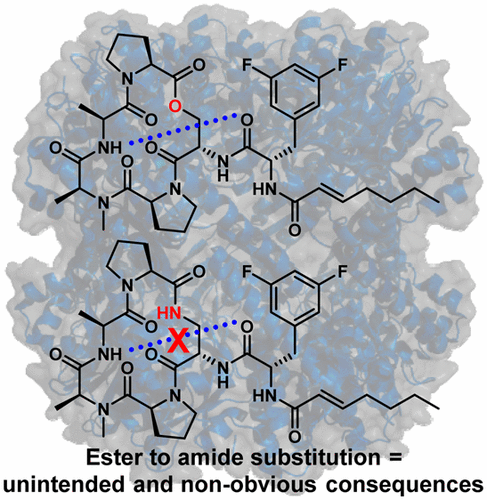
The acyldepsipeptide (ADEP) antibiotics operate through a clinically unexploited mechanism of action and thus have attracted attention from several antibacterial development groups. The ADEP scaffold is synthetically tractable, and deep-seated modifications have produced extremely potent antibacterial leads against Gram-positive pathogens. Although newly identified ADEP analogs demonstrate remarkable antibacterial activity against bacterial isolates and in mouse models of bacterial infections, stability issues pertaining to the depsipeptide core remain. To date, no study has been reported on the natural ADEP scaffold that evaluates the sole importance of the macrocyclic linkage on target engagement, molecular conformation, and bioactivity. To address this gap in ADEP structure–activity relationships, we synthesized three ADEP analogs that only differ in the linkage motif (i.e., ester, amide, and N-methyl amide) and provide a side-by-side comparison of conformational behavior and biological activity. We demonstrate that while replacement of the naturally occurring ester linkage with a secondary amide maintains in vitro biochemical activity, this simple substitution results in a significant drop in whole-cell activity. This study provides direct evidence that ester to amide linkage substitution is unlikely to provide a reasonable solution for ADEP instability.
中文翻译:

二肽取代对抗菌肽二肽ClpP活化活性的影响
酰基肽肽(ADEP)抗生素通过临床上尚未开发的作用机制起作用,因此引起了数个抗菌开发小组的关注。ADEP支架在合成上易于处理,并且深层修饰已产生针对革兰氏阳性病原体的极强抗菌力。尽管新近鉴定出的ADEP类似物对细菌分离物和在细菌感染的小鼠模型中显示出显着的抗菌活性,但与十肽核心有关的稳定性问题仍然存在。迄今为止,尚未有关于天然ADEP支架的研究报告,该支架评估大环键对靶标参与,分子构象和生物活性的唯一重要性。为了解决ADEP结构与活动之间的差距,N-甲基酰胺),并提供构象行为和生物活性的并排比较。我们证明,虽然用仲酰胺取代天然存在的酯键可保持体外生化活性,但这种简单的取代导致全细胞活性显着下降。这项研究提供了直接的证据,表明酯与酰胺键的取代不太可能为ADEP的不稳定提供合理的解决方案。
更新日期:2017-10-27
中文翻译:

二肽取代对抗菌肽二肽ClpP活化活性的影响
酰基肽肽(ADEP)抗生素通过临床上尚未开发的作用机制起作用,因此引起了数个抗菌开发小组的关注。ADEP支架在合成上易于处理,并且深层修饰已产生针对革兰氏阳性病原体的极强抗菌力。尽管新近鉴定出的ADEP类似物对细菌分离物和在细菌感染的小鼠模型中显示出显着的抗菌活性,但与十肽核心有关的稳定性问题仍然存在。迄今为止,尚未有关于天然ADEP支架的研究报告,该支架评估大环键对靶标参与,分子构象和生物活性的唯一重要性。为了解决ADEP结构与活动之间的差距,N-甲基酰胺),并提供构象行为和生物活性的并排比较。我们证明,虽然用仲酰胺取代天然存在的酯键可保持体外生化活性,但这种简单的取代导致全细胞活性显着下降。这项研究提供了直接的证据,表明酯与酰胺键的取代不太可能为ADEP的不稳定提供合理的解决方案。











































 京公网安备 11010802027423号
京公网安备 11010802027423号