Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2017-10-26 , DOI: 10.1016/j.bmcl.2017.10.059 Wen-Long Wang , Xiao-Yu Chen , Ya Gao , Li-Xin Gao , Li Sheng , Jingyu Zhu , Lei Xu , Zhen-Zhong Ding , Chao Zhang , Jing-Ya Li , Jia Li , Yu-Bo Zhou
|
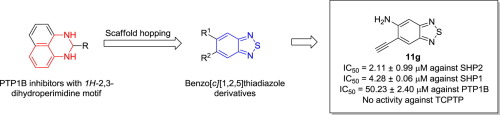
The Src homology-2 domain containing protein tyrosine phosphatase-2 (SHP2) is an oncogenic phosphatase linked to various kinds of cancers. Consequently, SHP2 has emerged as a promising target for novel anti-cancer agents. Using scaffold-hopping strategy, a series of benzo[c][1,2,5]thiadiazole derivatives was designed from PTP1B inhibitors with 1H-2,3-Dihydroperimidine motif, synthesized and evaluated their biological activities against PTP1B and SHP2. Among them, the representative compound 11g displayed SHP2 inhibitory activity with IC50 of 2.11 ± 0.99 μM, exhibited 2.02-fold and 25-fold selectivity for SHP2 over SHP1 and PTP1B respectively and had no visible activity against TCPTP. These preliminary results could provide a possible opportunity for the development of novel SHP2 inhibitors with optimal potency and improved pharmacological properties.
中文翻译:

苯并[ c ] [1,2,5]噻二唑衍生物:一类新的强效Src同源性2结构域,含蛋白酪氨酸磷酸酶2(SHP2)抑制剂
包含蛋白酪氨酸磷酸酶2(SHP2)的Src同源2结构域是一种与多种癌症相关的致癌磷酸酶。因此,SHP2已成为新型抗癌药物的有希望的靶标。运用支架跳跃策略,从具有1H -2,3-二氢哌啶基序的PTP1B抑制剂中设计了一系列苯并[ c ] [1,2,5]噻二唑衍生物,合成并评价了它们对PTP1B和SHP2的生物学活性。其中,代表性化合物11g对SHP2的抑制活性为IC 50相对于SHP1和PTP1B,SHP2的SHP2选择性为2.11±0.99μM,分别具有2.02倍和25倍的选择性,并且对TCPTP没有可见活性。这些初步结果可能为开发具有最佳效价和改善药理特性的新型SHP2抑制剂提供可能的机会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号