当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Small Molecule Antagonists of the Interaction between the Histone Deacetylase 6 Zinc-Finger Domain and Ubiquitin
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2017-10-27 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00933 Rachel J. Harding 1 , Renato Ferreira de Freitas 1 , Patrick Collins 2 , Ivan Franzoni 3 , Mani Ravichandran 1 , Hui Ouyang 4 , Kevin A. Juarez-Ornelas 3 , Mark Lautens 3 , Matthieu Schapira 1, 5 , Frank von Delft 2, 6 , Vjayaratnam Santhakumar 1 , Cheryl H. Arrowsmith 1
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2017-10-27 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00933 Rachel J. Harding 1 , Renato Ferreira de Freitas 1 , Patrick Collins 2 , Ivan Franzoni 3 , Mani Ravichandran 1 , Hui Ouyang 4 , Kevin A. Juarez-Ornelas 3 , Mark Lautens 3 , Matthieu Schapira 1, 5 , Frank von Delft 2, 6 , Vjayaratnam Santhakumar 1 , Cheryl H. Arrowsmith 1
Affiliation
|
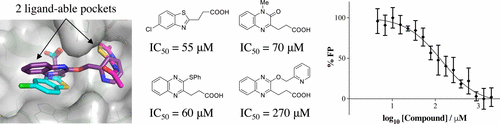
Inhibitors of HDAC6 have attractive potential in numerous cancers. HDAC6 inhibitors to date target the catalytic domains, but targeting the unique zinc-finger ubiquitin-binding domain (Zf-UBD) of HDAC6 may be an attractive alternative strategy. We developed X-ray crystallography and biophysical assays to identify and characterize small molecules capable of binding to the Zf-UBD and competing with ubiquitin binding. Our results revealed two adjacent ligand-able pockets of HDAC6 Zf-UBD and the first functional ligands for this domain.
中文翻译:

组蛋白脱乙酰基酶6锌指结构域和泛素之间相互作用的小分子拮抗剂。
HDAC6抑制剂在多种癌症中具有诱人的潜力。迄今为止,HDAC6抑制剂靶向催化结构域,但靶向HDAC6独特的锌指泛素结合结构域(Zf-UBD)可能是一种有吸引力的替代策略。我们开发了X射线晶体学和生物物理测定法,以鉴定和表征能够与Zf-UBD结合并与泛素结合竞争的小分子。我们的结果揭示了HDAC6 Zf-UBD的两个相邻的可配体口袋和该域的第一个功能性配体。
更新日期:2017-10-27
中文翻译:

组蛋白脱乙酰基酶6锌指结构域和泛素之间相互作用的小分子拮抗剂。
HDAC6抑制剂在多种癌症中具有诱人的潜力。迄今为止,HDAC6抑制剂靶向催化结构域,但靶向HDAC6独特的锌指泛素结合结构域(Zf-UBD)可能是一种有吸引力的替代策略。我们开发了X射线晶体学和生物物理测定法,以鉴定和表征能够与Zf-UBD结合并与泛素结合竞争的小分子。我们的结果揭示了HDAC6 Zf-UBD的两个相邻的可配体口袋和该域的第一个功能性配体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号