当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of Thiophene[3,2-d]pyrimidine Derivatives as Potent HIV-1 NNRTIs Targeting the Tolerant Region I of NNIBP
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2017-10-26 00:00:00 , DOI: 10.1021/acsmedchemlett.7b00361 Dongwei Kang 1 , Xiao Ding 1 , Gaochan Wu 1 , Zhipeng Huo 1 , Zhongxia Zhou 1 , Tong Zhao 1 , Da Feng 1 , Zhao Wang 1 , Ye Tian 1 , Dirk Daelemans 2 , Erik De Clercq 2 , Christophe Pannecouque 2 , Peng Zhan 1 , Xinyong Liu 1
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2017-10-26 00:00:00 , DOI: 10.1021/acsmedchemlett.7b00361 Dongwei Kang 1 , Xiao Ding 1 , Gaochan Wu 1 , Zhipeng Huo 1 , Zhongxia Zhou 1 , Tong Zhao 1 , Da Feng 1 , Zhao Wang 1 , Ye Tian 1 , Dirk Daelemans 2 , Erik De Clercq 2 , Christophe Pannecouque 2 , Peng Zhan 1 , Xinyong Liu 1
Affiliation
|
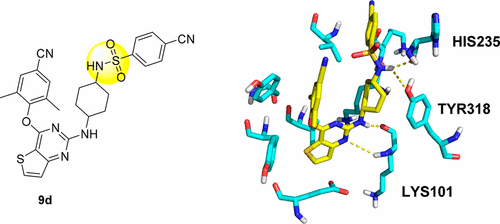
Our previous studies led us to conclude that thiophene[3,2-d]pyrimidine is a promising scaffold for diarylpyrimidine (DAPY)-type anti-HIV agents with potent activity against resistance-associated human immunodeficiency virus (HIV) variants (J. Med. Chem. 2016, 59, 7991–8007; J. Med. Chem. 2017, 60, 4424–4443). In the present study, we designed and synthesized a series of thiophenepyrimidine derivatives with various substituents in the right wing region of the structure with the aim of developing new interactions with the tolerant region I of the binding pocket of the HIV-1 non-nucleoside reverse transcriptase (NNRTI), and we evaluated their activity against a panel of mutant HIV-1 strains. All the derivatives exhibited moderate to excellent potency against wild-type (WT) HIV-1 in MT-4 cells. Among them, sulfonamide compounds 9b and 9d were single-figure-nanomolar inhibitors with EC50 values of 9.2 and 7.1 nM, respectively. Indeed, 9a and 9d were effective against the whole viral panel except RES056. Notably, both compounds showed potent antiviral activity against K103N (EC50 = 0.032 and 0.070 μM) and E138K (EC50 = 0.035 and 0.045 μM, respectively). Furthermore, 9a and 9d exhibited high affinity for WT HIV-1 RT (IC50 = 1.041 and 1.138 μM, respectively) and acted as classical NNRT inhibitors (NNRTIs). These results are expected to be helpful in the design of thiophenepyrimidine-based NNRTIs with more potent activity against HIV strains with RT mutations.
中文翻译:

发现噻吩[3,2- d ]嘧啶衍生物作为有效的HIV-1 NNRTIs,靶向NNIBP的耐受区I
我们以前的研究使我们得出结论,噻吩[3,2- d ]嘧啶是二芳基嘧啶(DAPY)型抗HIV药物的有前途的支架,其对与耐药相关的人类免疫缺陷病毒(HIV)变异体具有有效的活性(J. Med 。化学。2016,59,7991-8007; J。医学化学。2017年,60,4424–4443)。在本研究中,我们设计和合成了一系列在结构的右翼区域带有各种取代基的噻吩并嘧啶衍生物,目的是与HIV-1非核苷反向结合口袋的耐受区域I产生新的相互作用。转录酶(NNRTI),我们评估了它们对一组突变HIV-1菌株的活性。所有衍生物均对MT-4细胞中的野生型(WT)HIV-1表现出中等至极强的效力。其中,磺酰胺化合物9b和9d是单图纳米摩尔抑制剂,EC 50值分别为9.2和7.1 nM。确实是9a和9d除RES056外,对整个病毒组均有效。值得注意的是,两种化合物均显示出对K103N(EC 50 = 0.032和0.070μM)和E138K(EC 50 = 0.035和0.045μM)的有效抗病毒活性。此外,9a和9d对WT HIV-1 RT表现出高亲和力(分别为IC 50 = 1.041和1.138μM),并充当经典NNRT抑制剂(NNRTIs)。预期这些结果将有助于设计基于噻吩并嘧啶的NNRTI,对具有RT突变的HIV菌株具有更强的活性。
更新日期:2017-10-26
中文翻译:

发现噻吩[3,2- d ]嘧啶衍生物作为有效的HIV-1 NNRTIs,靶向NNIBP的耐受区I
我们以前的研究使我们得出结论,噻吩[3,2- d ]嘧啶是二芳基嘧啶(DAPY)型抗HIV药物的有前途的支架,其对与耐药相关的人类免疫缺陷病毒(HIV)变异体具有有效的活性(J. Med 。化学。2016,59,7991-8007; J。医学化学。2017年,60,4424–4443)。在本研究中,我们设计和合成了一系列在结构的右翼区域带有各种取代基的噻吩并嘧啶衍生物,目的是与HIV-1非核苷反向结合口袋的耐受区域I产生新的相互作用。转录酶(NNRTI),我们评估了它们对一组突变HIV-1菌株的活性。所有衍生物均对MT-4细胞中的野生型(WT)HIV-1表现出中等至极强的效力。其中,磺酰胺化合物9b和9d是单图纳米摩尔抑制剂,EC 50值分别为9.2和7.1 nM。确实是9a和9d除RES056外,对整个病毒组均有效。值得注意的是,两种化合物均显示出对K103N(EC 50 = 0.032和0.070μM)和E138K(EC 50 = 0.035和0.045μM)的有效抗病毒活性。此外,9a和9d对WT HIV-1 RT表现出高亲和力(分别为IC 50 = 1.041和1.138μM),并充当经典NNRT抑制剂(NNRTIs)。预期这些结果将有助于设计基于噻吩并嘧啶的NNRTI,对具有RT突变的HIV菌株具有更强的活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号