PLoS Pathogens ( IF 5.5 ) Pub Date : 2017-10-25 , DOI: 10.1371/journal.ppat.1006699 Lena Böhm , Sanda Torsin , Su Hlaing Tint , Marie Therese Eckstein , Tobias Ludwig , J. Christian Pérez
|
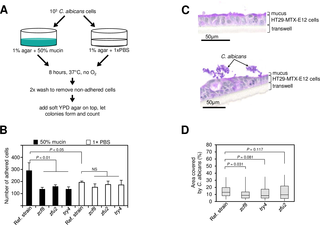
Many microorganisms that cause systemic, life-threatening infections in humans reside as harmless commensals in our digestive tract. Yet little is known about the biology of these microbes in the gut. Here, we visualize the interface between the human commensal and pathogenic fungus Candida albicans and the intestine of mice, a surrogate host. Because the indigenous mouse microbiota restricts C. albicans settlement, we compared the patterns of colonization in the gut of germ free and antibiotic-treated conventionally raised mice. In contrast to the heterogeneous morphologies found in the latter, we establish that in germ free animals the fungus almost uniformly adopts the yeast cell form, a proxy of its commensal state. By screening a collection of C. albicans transcription regulator deletion mutants in gnotobiotic mice, we identify several genes previously unknown to contribute to in vivo fitness. We investigate three of these regulators—ZCF8, ZFU2 and TRY4—and show that indeed they favor the yeast form over other morphologies. Consistent with this finding, we demonstrate that genetically inducing non-yeast cell morphologies is detrimental to the fitness of C. albicans in the gut. Furthermore, the identified regulators promote adherence of the fungus to a surface covered with mucin and to mucus-producing intestinal epithelial cells. In agreement with this result, histology sections indicate that C. albicans dwells in the murine gut in close proximity to the mucus layer. Thus, our findings reveal a set of regulators that endows C. albicans with the ability to endure in the intestine through multiple mechanisms.
中文翻译:

真菌白色念珠菌的酵母形式可促进其在gnotobiotic小鼠肠道中的持久性
许多导致人类系统性威胁生命的感染的微生物作为无害的食物存在于我们的消化道中。对于肠道中这些微生物的生物学知之甚少。在这里,我们可视化人类共生和致病性真菌白色念珠菌与小鼠肠道(替代宿主)之间的界面。因为土著微生物鼠标限制Ç。在白色念珠菌的定居中,我们比较了无菌和经抗生素处理的常规饲养小鼠肠道中的定殖模式。与后者中发现的异质形态相反,我们确定在无菌动物中,真菌几乎均匀地采用酵母细胞形式,即其共生状态的代表。通过筛选Ç。白色素转录调节子缺失突变体在致病菌小鼠中,我们确定了以前未知的几个基因,它们对体内适应性有贡献。我们研究了ZCF8,ZFU2和TRY4这三个调控因子,并表明它们确实比其他形态更倾向于酵母形式。与这个发现一致,我们证明了遗传诱导非酵母细胞的形态对C的适应性是有害的。白色的在肠子里。此外,已鉴定的调节剂促进了真菌对粘蛋白覆盖的表面和产生粘液的肠上皮细胞的粘附。与此结果一致,组织切片显示,Ç。白色念珠菌生活在靠近粘液层的鼠肠内。因此,我们的发现揭示了一组赋予C的监管机构。具有通过多种机制在肠道中忍受的能力的白色念珠菌。











































 京公网安备 11010802027423号
京公网安备 11010802027423号