当前位置:
X-MOL 学术
›
Sustain. Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A hybrid catalyst of Pt/CoNiO2 on carbon nanotubes and its synergetic effect towards remarkable ethanol electro-oxidation in alkaline media†
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2017-10-25 00:00:00 , DOI: 10.1039/c7se00392g Tingting Tang 1, 2, 3, 4, 5 , Qiuping Gan 1, 2, 3, 4, 5 , Xiaohui Guo 1, 2, 3, 4, 5 , Hailin Dong 6, 7, 8, 9 , Jifang Zhang 1, 2, 3, 4, 5 , Yanchun Zhao 1, 2, 3, 4, 5 , Jianniao Tian 1, 2, 3, 4, 5 , Xiulin Yang 1, 2, 3, 4, 5
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2017-10-25 00:00:00 , DOI: 10.1039/c7se00392g Tingting Tang 1, 2, 3, 4, 5 , Qiuping Gan 1, 2, 3, 4, 5 , Xiaohui Guo 1, 2, 3, 4, 5 , Hailin Dong 6, 7, 8, 9 , Jifang Zhang 1, 2, 3, 4, 5 , Yanchun Zhao 1, 2, 3, 4, 5 , Jianniao Tian 1, 2, 3, 4, 5 , Xiulin Yang 1, 2, 3, 4, 5
Affiliation
|
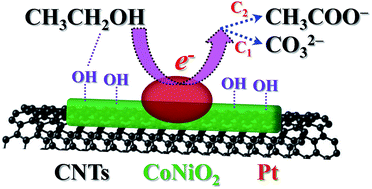
Herein, a hybrid catalyst of Pt/CoNiO2 on carbon nanotubes (Pt/CoNiO2–CNTs) has been successfully synthesized by a facile and cost-effective method, and its crystal structures, chemical valence states, and morphologies have been characterized in detail. CO stripping voltammograms reveal that the adsorbed COads on the active sites of the Pt/CoNiO2–CNT catalyst is easily oxidized at a lower potential (−0.60 V) as compared to the Pt particles on rGO (−0.35 V) and acid-treated CNTs (−0.36 V). Cyclic voltammograms demonstrate that the designed Pt/CoNiO2–CNT catalyst possesses an ultrahigh electrocatalytic activity (1136.2 mA mgPt−1) for ethanol oxidation, which is 5.1 and 3.0 times higher than that of Pt/rGO (221.6 mA mgPt−1) and Pt/CNTs (375.4 mA mgPt−1), respectively. The Tafel plot of Pt/CoNiO2–CNTs is 205 mV dec−1, indicating much faster reaction kinetics than that of the compared catalysts. In addition, the outstanding long-term stability indicates that the designed Pt/CoNiO2–CNT catalyst exhibits expected application prospects in direct alkaline ethanol fuel cells. Moreover, the catalytic mechanism of the hybrid Pt/CoNiO2–CNTs has been proposed and discussed via C2 and C1 pathways with respect to the final products for CH3COO− and CO32−, respectively.
中文翻译:

碳纳米管上 Pt / CoNiO 2的杂化催化剂及其在碱性介质中对显着乙醇电氧化的协同作用†
在此,已通过一种简便且经济高效的方法成功地合成了碳纳米管上的Pt / CoNiO 2杂化催化剂(Pt / CoNiO 2 -CNTs),并详细表征了其晶体结构,化学价态和形态。 。CO剥离伏安表明被吸附的CO的广告在Pt / CoNiO的活性位点2 -CNT催化剂在相比于上RGO(-0.35 V)和酸的Pt粒子低的电势(-0.60 V)容易氧化处理的CNT(-0.36 V)。循环伏安图表明,设计的Pt / CoNiO 2 -CNT催化剂具有超高的电催化活性(1136.2 mA mg Pt -1)进行乙醇氧化,分别是Pt / rGO(221.6 mA mg Pt -1)和Pt / CNTs(375.4 mA mg Pt -1)的5.1和3.0倍。Pt / CoNiO 2 -CNTs的Tafel图为205 mV dec -1,表明反应动力学比比较的催化剂快得多。此外,出色的长期稳定性表明,设计的Pt / CoNiO 2 -CNT催化剂在直接碱性乙醇燃料电池中具有预期的应用前景。此外,关于CH 3 COO的最终产物,还通过C2和C1途径提出并讨论了混合Pt / CoNiO 2 -CNTs的催化机理。-和CO 3 2-。
更新日期:2017-10-25
中文翻译:

碳纳米管上 Pt / CoNiO 2的杂化催化剂及其在碱性介质中对显着乙醇电氧化的协同作用†
在此,已通过一种简便且经济高效的方法成功地合成了碳纳米管上的Pt / CoNiO 2杂化催化剂(Pt / CoNiO 2 -CNTs),并详细表征了其晶体结构,化学价态和形态。 。CO剥离伏安表明被吸附的CO的广告在Pt / CoNiO的活性位点2 -CNT催化剂在相比于上RGO(-0.35 V)和酸的Pt粒子低的电势(-0.60 V)容易氧化处理的CNT(-0.36 V)。循环伏安图表明,设计的Pt / CoNiO 2 -CNT催化剂具有超高的电催化活性(1136.2 mA mg Pt -1)进行乙醇氧化,分别是Pt / rGO(221.6 mA mg Pt -1)和Pt / CNTs(375.4 mA mg Pt -1)的5.1和3.0倍。Pt / CoNiO 2 -CNTs的Tafel图为205 mV dec -1,表明反应动力学比比较的催化剂快得多。此外,出色的长期稳定性表明,设计的Pt / CoNiO 2 -CNT催化剂在直接碱性乙醇燃料电池中具有预期的应用前景。此外,关于CH 3 COO的最终产物,还通过C2和C1途径提出并讨论了混合Pt / CoNiO 2 -CNTs的催化机理。-和CO 3 2-。











































 京公网安备 11010802027423号
京公网安备 11010802027423号