当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Recellularization of decellularized adipose tissue-derived stem cells: role of the cell-secreted extracellular matrix in cellular differentiation†
Biomaterials Science ( IF 5.8 ) Pub Date : 2017-10-25 00:00:00 , DOI: 10.1039/c7bm00695k V. Guneta 1, 2, 3 , Z. Zhou 3, 4, 5 , N. S. Tan 2, 3, 6, 7, 8 , S. Sugii 3, 4, 5 , M. T. C. Wong 3, 9, 10, 11 , C. Choong 1, 2, 3
Biomaterials Science ( IF 5.8 ) Pub Date : 2017-10-25 00:00:00 , DOI: 10.1039/c7bm00695k V. Guneta 1, 2, 3 , Z. Zhou 3, 4, 5 , N. S. Tan 2, 3, 6, 7, 8 , S. Sugii 3, 4, 5 , M. T. C. Wong 3, 9, 10, 11 , C. Choong 1, 2, 3
Affiliation
|
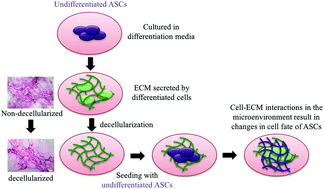
Adipose-derived stem cells (ASCs) are found in a location within the adipose tissue known as the stem cell niche. The ASCs in the niche are maintained in the quiescent state, and upon exposure to various microenvironmental triggers are prompted to undergo proliferation or differentiation. These microenvironmental triggers also modulate the extracellular matrix (ECM), which interacts with the cells through the cytoskeleton and induces downstream events inside the cells that bring about a change in cell behaviour. In response to these changes, the cells remodel the ECM, which will differ according to the type of tissue being formed by the cells. As the ECM itself plays an important role in the regulation of cellular differentiation, this study aims to explore the role of the cell-secreted ECM at various stages of differentiation of stem cells in triggering the differentiation of ASCs. To this end, the ASCs cultured in proliferation, osteogenic and adipogenic media were decellularized and the secreted ECM was characterized. Overall, it was found that osteo-differentiated ASCs produced higher amounts of collagen and glycosaminoglycans (GAG) compared to the undifferentiated and adipo-differentiated ASCs. The two types of differentiated ECMs were subsequently shown to trigger initial but not terminal differentiation of ASCs into osteo- and adipo-lineages respectively, as indicated by the upregulation of lineage specific markers. In addition, integrin subunits alpha (α) 6 and integrin beta (β) 1 were found to be produced by ASCs cultured on cell-secreted ECM-coated substrates, suggesting that the integrins α6 and β1 play an instrumental role in cell–ECM interactions. Taken together, this study demonstrates the importance of the ECM in cellular fate decisions and how ECM-coated substrates can potentially be used for various tissue engineering applications.
中文翻译:

去细胞脂肪组织干细胞的再细胞化:细胞分泌的细胞外基质在细胞分化中的作用†
脂肪干细胞(ASC)位于脂肪组织内称为干细胞小生境的位置。生态位中的ASC保持静止状态,一旦暴露于各种微环境触发条件下,它们就会发生增殖或分化。这些微环境触发因素还调节细胞外基质(ECM),该基质通过细胞骨架与细胞相互作用,并诱导细胞内下游事件,从而引起细胞行为的改变。响应于这些变化,细胞重塑ECM,ECM将根据细胞形成的组织类型而有所不同。由于ECM本身在细胞分化的调节中起着重要作用,这项研究旨在探讨干细胞分化各个阶段中分泌细胞的ECM在触发ASC分化中的作用。为此,将在增殖,成骨和成脂培养基中培养的ASC脱细胞,并对分泌的ECM进行表征。总体而言,发现与未分化和脂肪分化的ASC相比,经骨分化的ASC产生更高数量的胶原蛋白和糖胺聚糖(GAG)。随后显示出两种分化的ECM,分别触发ASC的初始分化,而不是终末分化,分别触发成骨和脂肪谱系,这由谱系特异性标志物的上调所表明。此外,发现整合素亚基α(α)6和整合素β(β)1是由在分泌细胞的ECM涂层基质上培养的ASC产生的,提示整合素α6和β1在细胞-ECM相互作用中起重要作用。两者合计,这项研究证明了ECM在细胞命运决定中的重要性,以及如何将ECM涂层基质潜在地用于各种组织工程应用。
更新日期:2017-10-25
中文翻译:

去细胞脂肪组织干细胞的再细胞化:细胞分泌的细胞外基质在细胞分化中的作用†
脂肪干细胞(ASC)位于脂肪组织内称为干细胞小生境的位置。生态位中的ASC保持静止状态,一旦暴露于各种微环境触发条件下,它们就会发生增殖或分化。这些微环境触发因素还调节细胞外基质(ECM),该基质通过细胞骨架与细胞相互作用,并诱导细胞内下游事件,从而引起细胞行为的改变。响应于这些变化,细胞重塑ECM,ECM将根据细胞形成的组织类型而有所不同。由于ECM本身在细胞分化的调节中起着重要作用,这项研究旨在探讨干细胞分化各个阶段中分泌细胞的ECM在触发ASC分化中的作用。为此,将在增殖,成骨和成脂培养基中培养的ASC脱细胞,并对分泌的ECM进行表征。总体而言,发现与未分化和脂肪分化的ASC相比,经骨分化的ASC产生更高数量的胶原蛋白和糖胺聚糖(GAG)。随后显示出两种分化的ECM,分别触发ASC的初始分化,而不是终末分化,分别触发成骨和脂肪谱系,这由谱系特异性标志物的上调所表明。此外,发现整合素亚基α(α)6和整合素β(β)1是由在分泌细胞的ECM涂层基质上培养的ASC产生的,提示整合素α6和β1在细胞-ECM相互作用中起重要作用。两者合计,这项研究证明了ECM在细胞命运决定中的重要性,以及如何将ECM涂层基质潜在地用于各种组织工程应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号