当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A versatile rhodium(III) catalyst for direct acyloxylation of aryl and alkenyl C–H bonds with carboxylic acids
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-10-16 00:00:00 , DOI: 10.1039/c7qo00844a Changjun Chen 1, 2, 3, 4 , Yixiao Pan 1, 2, 3, 4 , Haoqiang Zhao 1, 2, 3, 4 , Xin Xu 1, 2, 3, 4 , Jianbin Xu 1, 2, 3, 4 , Zongyao Zhang 1, 2, 3, 4 , Siqi Xi 1, 2, 3, 4 , Lijin Xu 1, 2, 3, 4 , Huanrong Li 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-10-16 00:00:00 , DOI: 10.1039/c7qo00844a Changjun Chen 1, 2, 3, 4 , Yixiao Pan 1, 2, 3, 4 , Haoqiang Zhao 1, 2, 3, 4 , Xin Xu 1, 2, 3, 4 , Jianbin Xu 1, 2, 3, 4 , Zongyao Zhang 1, 2, 3, 4 , Siqi Xi 1, 2, 3, 4 , Lijin Xu 1, 2, 3, 4 , Huanrong Li 1, 2, 3, 4
Affiliation
|
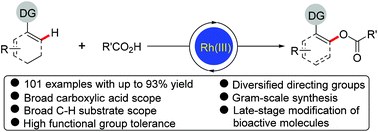
Rh(III)-Catalyzed highly regioselective direct acyloxylation of sp2 C–H bonds with carboxylic acids has been developed. The catalytic system consisting of a cationic Rh(III) complex and a silver oxidant allowed a variety of arenes and alkenes bearing different directing groups (not limited to strongly coordinating N-heterocyclic directing groups) to undergo efficient acyloxylation with a broad range of readily available alkyl, alkenyl and aryl carboxylic acids, and a number of valuable functional groups in both coupling partners were well tolerated in the reaction regardless of their electronic properties and positions. This method provides a straightforward and convenient access to various valuable acyloxylated products. Furthermore, the synthetic utility of this protocol was demonstrated by the later-stage functionalization and modification of biologically active compounds. Mechanistic studies reveal the involvement of five-membered rhodacycles as key intermediates.
中文翻译:

一种多功能的铑(III)催化剂,用于将芳基和烯基CH键与羧酸直接进行酰氧基化
Rh(III)催化sp 2 C–H键与羧酸的高度区域选择性直接酰氧基化反应已经开发出来。阳离子Rh(III)组成的催化体系)配合物和银氧化剂可使带有不同指导基团(不限于强配位N-杂环指导基团)的各种芳烃和烯烃与各种现成的烷基,烯基和芳基羧酸进行有效的酰氧基化反应,并且不论其电子性质和位置如何,两个偶联伙伴中有价值的官能团的数量都被很好地耐受。该方法提供了直接和方便的途径来获得各种有价值的酰氧基化产物。此外,该协议的合成效用通过生物活性化合物的后期功能化和修饰得到了证明。机理研究表明,五元rhodocycles作为关键中间体参与其中。
更新日期:2017-10-25
中文翻译:

一种多功能的铑(III)催化剂,用于将芳基和烯基CH键与羧酸直接进行酰氧基化
Rh(III)催化sp 2 C–H键与羧酸的高度区域选择性直接酰氧基化反应已经开发出来。阳离子Rh(III)组成的催化体系)配合物和银氧化剂可使带有不同指导基团(不限于强配位N-杂环指导基团)的各种芳烃和烯烃与各种现成的烷基,烯基和芳基羧酸进行有效的酰氧基化反应,并且不论其电子性质和位置如何,两个偶联伙伴中有价值的官能团的数量都被很好地耐受。该方法提供了直接和方便的途径来获得各种有价值的酰氧基化产物。此外,该协议的合成效用通过生物活性化合物的后期功能化和修饰得到了证明。机理研究表明,五元rhodocycles作为关键中间体参与其中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号