当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Stereospecific C–H activation as a key step for the asymmetric synthesis of various biologically active cyclopropanes
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1039/c7qo00737j S. Jerhaoui 1, 2, 3, 4, 5 , P. Poutrel 1, 2, 3, 4, 5 , J.-P. Djukic 2, 5, 6, 7, 8 , J. Wencel-Delord 1, 2, 3, 4, 5 , F. Colobert 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-09-19 00:00:00 , DOI: 10.1039/c7qo00737j S. Jerhaoui 1, 2, 3, 4, 5 , P. Poutrel 1, 2, 3, 4, 5 , J.-P. Djukic 2, 5, 6, 7, 8 , J. Wencel-Delord 1, 2, 3, 4, 5 , F. Colobert 1, 2, 3, 4, 5
Affiliation
|
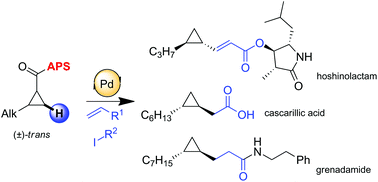
A rapid and efficient synthesis of hoshinolactam, an enantiopure cyclopropane containing natural product, is described. This strategy is based on stereospecific C(sp3)–H activation via unprecedented olefination on the cyclopropane core. The use of a stereogenic and easily recyclable directing group (S)-2-(p-tolylsulfinyl)aniline (APS) which was originally developed by our group allows obtention of diastereomerically pure products in high yields. Optically pure cyclopropanes are subsequently converted into enantiomerically pure targeted natural products. Furthermore a closely related synthetic scenario is employed to build up precursors of grenadamide and cascarillic acid.
中文翻译:

立体定向C–H活化是各种生物活性环丙烷不对称合成的关键步骤
描述了快速和有效地合成鼻内酰胺(一种对映体纯的含环丙烷的天然产物)。该策略基于通过环丙烷核上空前的烯化反应的立体特异性C(sp 3)–H活化。使用最初由我们小组开发的立体异构且易于回收的导向基团(S)-2-(对甲苯磺酰亚胺基)苯胺(APS),可以以高收率获得非对映体纯的产物。旋光纯的环丙烷随后被转化为对映体纯的目标天然产物。此外,采用了密切相关的合成方案来构建格林纳丁酰胺和卡斯卡利酸的前体。
更新日期:2017-10-25
中文翻译:

立体定向C–H活化是各种生物活性环丙烷不对称合成的关键步骤
描述了快速和有效地合成鼻内酰胺(一种对映体纯的含环丙烷的天然产物)。该策略基于通过环丙烷核上空前的烯化反应的立体特异性C(sp 3)–H活化。使用最初由我们小组开发的立体异构且易于回收的导向基团(S)-2-(对甲苯磺酰亚胺基)苯胺(APS),可以以高收率获得非对映体纯的产物。旋光纯的环丙烷随后被转化为对映体纯的目标天然产物。此外,采用了密切相关的合成方案来构建格林纳丁酰胺和卡斯卡利酸的前体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号