当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile synthesis of 1-aminoindoles via Rh(III)-catalysed intramolecular three-component annulation
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-08-07 00:00:00 , DOI: 10.1039/c7qo00541e Zi Yang 1, 2, 3, 4, 5 , Xing Lin 1, 2, 3, 4, 5 , Lianhui Wang 1, 2, 3, 4, 5 , Xiuling Cui 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-08-07 00:00:00 , DOI: 10.1039/c7qo00541e Zi Yang 1, 2, 3, 4, 5 , Xing Lin 1, 2, 3, 4, 5 , Lianhui Wang 1, 2, 3, 4, 5 , Xiuling Cui 1, 2, 3, 4, 5
Affiliation
|
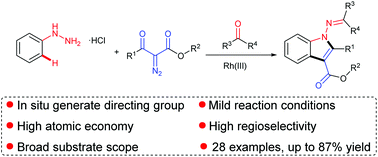
The facile synthesis of various 1-aminoindoles from readily available aryl hydrazines, diazo compounds and ketones via rhodium catalysed C–H activation has been developed. The reaction was carried out under mild reaction conditions using hydrazone as a directing group, which was formed by the in situ condensation of hydrazines and ketones. This protocol exhibits a broad substrate scope and step economy, releasing N2 and H2O as the byproducts, obviating the need for external oxidants and pre-functionalized substrates.
中文翻译:

通过Rh(III)催化的分子内三组分环化反应轻松合成1-氨基吲哚
已经开发了通过铑催化的C–H活化从易得的芳基肼,重氮化合物和酮轻松合成各种1-氨基吲哚的方法。该反应是在温和的反应条件下进行的,以为导向基团,hydr是通过肼和酮的原位缩合反应而形成的。该方案显示了广泛的底物范围和步骤经济性,释放出N 2和H 2 O作为副产物,从而消除了对外部氧化剂和预功能化底物的需求。
更新日期:2017-10-25
中文翻译:

通过Rh(III)催化的分子内三组分环化反应轻松合成1-氨基吲哚
已经开发了通过铑催化的C–H活化从易得的芳基肼,重氮化合物和酮轻松合成各种1-氨基吲哚的方法。该反应是在温和的反应条件下进行的,以为导向基团,hydr是通过肼和酮的原位缩合反应而形成的。该方案显示了广泛的底物范围和步骤经济性,释放出N 2和H 2 O作为副产物,从而消除了对外部氧化剂和预功能化底物的需求。











































 京公网安备 11010802027423号
京公网安备 11010802027423号