当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Post-synthetic diversification of pyrrole-fused benzosultams via trans-sulfonylations and reactions on the periphery of pyrrole
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-07-28 00:00:00 , DOI: 10.1039/c7qo00440k Joydev K. Laha 1, 2, 3 , Rohan A. Bhimpuria 1, 2, 3 , Aitha Manoj Kumar 1, 2, 3
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-07-28 00:00:00 , DOI: 10.1039/c7qo00440k Joydev K. Laha 1, 2, 3 , Rohan A. Bhimpuria 1, 2, 3 , Aitha Manoj Kumar 1, 2, 3
Affiliation
|
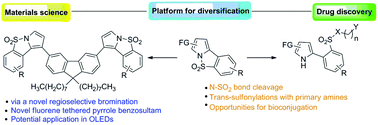
The chemistry (synthesis and reactions) of pyrrole-fused benzosultams has been the subject of this current investigation. Their workable access via palladium-catalyzed oxidative coupling has made this study feasible. The trans-sulfonylation in pyrrole-fused benzosultams provided access to otherwise unattainable (NH)-2-arylpyrroles containing an ortho-sulfonamide functionality. Other N–SO2 bond cleavages in pyrrole-fused benzosultams yielded synthetically useful (NH)-2-arylpyrroles containing other ortho-sulfonyl functionality. A terminal amine or hydroxyl functionality, ideally poised for bioconjugation, is another important feature of some of the synthesized (NH)-2-arylpyrroles. The rationally designed and synthesised novel fluorenes tethered with pyrrole-fused benzosultam could find applications as organic emitters in organic light-emitting devices (OLEDs).
中文翻译:

吡咯稠合的苯并磺胺类化合物的合成后通过反磺酰化和在吡咯周围的反应而多样化
吡咯稠合的苯并阿磺胺的化学(合成和反应)一直是本研究的主题。他们通过钯催化的氧化偶联的可行途径使这项研究可行。吡咯稠合的苯并苏磺酰胺中的反式磺酰化作用提供了获得原本无法获得的含有邻磺酰胺官能团的(NH)-2-芳基吡咯的途径。吡咯稠合的苯并阿苏咪唑中的其他N–SO 2键裂解产生了合成有用的(NH)-2-芳基吡咯,其中包含其他邻位-磺酰基官能团。理想地准备用于生物缀合的末端胺或羟基官能度是某些合成的(NH)-2-芳基吡咯的另一个重要特征。合理设计并合成的新型芴与吡咯稠合的苯并舒马坦束缚在一起,可以在有机发光器件(OLED)中用作有机发射体。
更新日期:2017-10-25
中文翻译:

吡咯稠合的苯并磺胺类化合物的合成后通过反磺酰化和在吡咯周围的反应而多样化
吡咯稠合的苯并阿磺胺的化学(合成和反应)一直是本研究的主题。他们通过钯催化的氧化偶联的可行途径使这项研究可行。吡咯稠合的苯并苏磺酰胺中的反式磺酰化作用提供了获得原本无法获得的含有邻磺酰胺官能团的(NH)-2-芳基吡咯的途径。吡咯稠合的苯并阿苏咪唑中的其他N–SO 2键裂解产生了合成有用的(NH)-2-芳基吡咯,其中包含其他邻位-磺酰基官能团。理想地准备用于生物缀合的末端胺或羟基官能度是某些合成的(NH)-2-芳基吡咯的另一个重要特征。合理设计并合成的新型芴与吡咯稠合的苯并舒马坦束缚在一起,可以在有机发光器件(OLED)中用作有机发射体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号