当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Vinyl/Phenyl Exchange Reaction within Vinyl Nickel Complexes Bearing Chelate [P, S]-Ligands
Organometallics ( IF 2.5 ) Pub Date : 2017-10-25 00:00:00 , DOI: 10.1021/acs.organomet.7b00671 Benjing Xue 1 , Hongjian Sun 1 , Shishuai Ren 1 , Xiaoyan Li 1 , Olaf Fuhr 2
Organometallics ( IF 2.5 ) Pub Date : 2017-10-25 00:00:00 , DOI: 10.1021/acs.organomet.7b00671 Benjing Xue 1 , Hongjian Sun 1 , Shishuai Ren 1 , Xiaoyan Li 1 , Olaf Fuhr 2
Affiliation
|
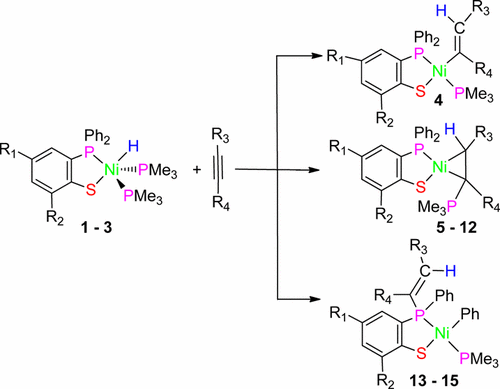
Three nickel(II) hydrides, [2-Ph2P(4-Me-C6H3)S]NiH(PMe3)2 (1), [2-Ph2P(6-Me3Si-C6H3)S]NiH(PMe3)2 (2), and [2-Ph2P(4-Me3Si -C6H3)S]NiH(PMe3)2 (3), were synthesized via S–H bond activation through the reaction of Ni(PMe3)4 with (2-diphenylphosphanyl)thiophenols. The reactions of nickel(II) hydrides (1–3) with different alkynes were investigated. Although the first step is the insertion of alkyne into the Ni–H bond for each reaction, different final products were isolated. Normal vinyl nickel complex [2-Ph2P(4-Me-C6H3)S]Ni(CPh═CH2)(PMe3) (4) was obtained by the reaction of phenylacetylene with 1. The nickelacyclopropane complexes [2-Ph2P(6-Me3Si-C6H3)S]Ni[Ph(PMe3)C–CH2] (5), [2-Ph2P(4-Me3Si-C6H3)S]Ni[Ph(PMe3)C–CH2] (6), [2-Ph2P(4-Me3-C6H3)S]Ni[Ph(PMe3)C–CHPh] (7), [2-Ph2P(6-Me3Si-C6H3)S]Ni[Ph(PMe3)C–CHPh] (8), [2-Ph2P(4-Me3Si-C6H3)S]Ni[Ph(PMe3)C–CHPh] (9), [2-Ph2P(4-Me-C6H3)S]Ni[Ph(PMe3)C–CHSiMe3] (10) or [2-Ph2P(4-Me-C6H3)S]Ni[Me3Si(PMe3)C–CHPh] (10), [2-Ph2P(6-Me3Si-C6H3)S]Ni[Ph(PMe3)C–CHSiMe3] (11) or [2-Ph2P(6-Me3Si-C6H3)S]Ni[Me3Si(PMe3)C–CHPh] (11), and [2-Ph2P(4-Me3Si-C6H3)S]Ni[Ph(PMe3)C–CHSiMe3] (12) or [2-Ph2P(4-Me3Si-C6H3)S]Ni[Me3Si(PMe3)C–CHPh] (12) containing a ylidic ligand were formed by the reaction of phenylacetylene, diphenylacetylene, and 1-phenyl-2-(trimethylsilyl)acetylene with 1, 2, and 3, respectively. The phenyl/vinyl exchange nickel(II) complexes [2-(Ph(CH2═CSiMe3)P(4-Me-C6H3)S]Ni(Ph)(PMe3) (13), [2-(Ph(CH2═CSiMe3)P((6-Me3Si-C6H3)S]Ni(Ph)(PMe3) (14), and [2-(Ph(CH2═CSiMe3)P((4-Me3Si-C6H3)S]Ni(Ph)(PMe3) (15) could be obtained by insertion of trimethylsilylacetylene into Ni–H bonds of 1, 2, and 3. To the best of our knowledge, this is a novel reaction type between alkyne and nickel hydride. The results indicate that whether increasing the electronegativity on the benzene ring or on the alkyne leads to the instability of the vinyl nickel complex, and is beneficial to the C–P reductive elimination to form nickelacyclopropane complexes or phenyl nickel complexes via vinyl/phenyl exchange reaction in the case of the more electronegative nickel center. All the nickel complexes were fully detected by IR, NMR and the molecular structures of complexes 1, 2, 7, 9, 13, and 14 were confirmed by single crystal X-ray diffraction.
中文翻译:

带有螯合物[P,S]-配体的乙烯基镍配合物中的乙烯基/苯基交换反应
三种氢化镍(II),[2-Ph 2 P(4-Me-C 6 H 3)S] NiH(PMe 3)2(1),[2-Ph 2 P(6-Me 3 Si-C 6)通过S合成H 3)S] NiH(PMe 3)2(2)和[2-Ph 2 P(4-Me 3 Si -C 6 H 3)S] NiH(PMe 3)2(3)。 Ni(PMe 3)4反应引起的-H键活化与(2-二苯基膦基)硫酚。镍的反应(II)氢化物(1 - 3)具有不同的炔烃进行了研究。尽管第一步是将炔烃插入每个反应的Ni–H键中,但分离出不同的最终产物。正常乙烯基镍络合物[2-PH 2 P(4-ME-C 6 H ^ 3)S]的Ni(CPh═CH 2)(PME 3)(4)通过苯乙炔与反应得到的1。镍环丙烷络合物[2-Ph 2 P(6-Me 3 Si-C 6 H 3)S] Ni [Ph(PMe 3)C–CH 2 ](5),[2-Ph 2 P(4-Me 3 Si-C 6 H 3)S] Ni [Ph(PMe 3)C–CH 2 ](6),[2-Ph 2 P(4-Me 3 -C 6 H 3)S] Ni [Ph(PMe 3)C–CHPh](7),[2-Ph 2 P(6-Me 3 Si-C 6 H 3)S] Ni [Ph(PMe 3) C–CHPh](8),[2-Ph 2 P(4-Me 3 Si-C 6 H 3)S] Ni [Ph(PMe 3)C–CHPh](9),[2-Ph 2 P(4-Me-C 6 H 3)S] Ni [Ph(PMe 3)C–CHSiMeMe 3 ](10)或[2-Ph 2 P(4-Me-C 6 H 3)S] Ni [Me 3 Si(PMe 3)C–CHPh](10),[2-Ph 2 P(6-Me 3 Si-C 6 H 3)S] Ni [Ph(PMe 3)C– CHSiMe 3 ](11)或[2-Ph 2 P(6-Me 3 Si-C 6 H 3)S] Ni [Me 3 Si(PMe 3)C–CHPh](11)和[2-Ph 2 P(4-Me 3 Si-C 6 H 3)S] Ni [Ph(PMe 3)C–CHSiMe 3 ](12)或[2-Ph通过苯乙炔,二苯乙炔和1-苯基的反应形成了含碘配体的2 P(4-Me 3 Si-C 6 H 3)S] Ni [Me 3 Si(PMe 3)C–CHPh](12) -2-(三甲基甲硅烷基)乙炔与1,2,和3,分别。苯基/乙烯基交换镍(II)配合物[2-(PH(CH 2 ═CSiMe3)P(4-ME-C 6 H ^ 3)S]的Ni(PH)(PME 3)(13),[2-(PH(CH 2 ═CSiMe 3)P((6-ME 3的Si-C 6 ħ 3)] S的Ni(PH)(PME 3)(14),和[2-(PH(CH 2 ═CSiMe 3)P((4-ME 3的Si-C 6 H ^ 3)S]的Ni(PH) (PME 3)(15)可通过三甲基硅烷基的插入来获得成的Ni-H键1,2,和3。据我们所知,这是炔烃与氢化镍之间的一种新型反应类型。结果表明,增加苯环或炔烃上的电负性会导致乙烯基镍配合物的不稳定性,并且有利于通过乙烯基/苯基交换反应进行C-P还原消除形成镍环杂环丙烷配合物或苯基镍配合物。如果镍中心的电负性更高。所有的镍配合物,用IR,NMR和复合的分子结构完全检测到1,2,7,9,13,和14是由单晶X射线衍射证实。
更新日期:2017-10-25
中文翻译:

带有螯合物[P,S]-配体的乙烯基镍配合物中的乙烯基/苯基交换反应
三种氢化镍(II),[2-Ph 2 P(4-Me-C 6 H 3)S] NiH(PMe 3)2(1),[2-Ph 2 P(6-Me 3 Si-C 6)通过S合成H 3)S] NiH(PMe 3)2(2)和[2-Ph 2 P(4-Me 3 Si -C 6 H 3)S] NiH(PMe 3)2(3)。 Ni(PMe 3)4反应引起的-H键活化与(2-二苯基膦基)硫酚。镍的反应(II)氢化物(1 - 3)具有不同的炔烃进行了研究。尽管第一步是将炔烃插入每个反应的Ni–H键中,但分离出不同的最终产物。正常乙烯基镍络合物[2-PH 2 P(4-ME-C 6 H ^ 3)S]的Ni(CPh═CH 2)(PME 3)(4)通过苯乙炔与反应得到的1。镍环丙烷络合物[2-Ph 2 P(6-Me 3 Si-C 6 H 3)S] Ni [Ph(PMe 3)C–CH 2 ](5),[2-Ph 2 P(4-Me 3 Si-C 6 H 3)S] Ni [Ph(PMe 3)C–CH 2 ](6),[2-Ph 2 P(4-Me 3 -C 6 H 3)S] Ni [Ph(PMe 3)C–CHPh](7),[2-Ph 2 P(6-Me 3 Si-C 6 H 3)S] Ni [Ph(PMe 3) C–CHPh](8),[2-Ph 2 P(4-Me 3 Si-C 6 H 3)S] Ni [Ph(PMe 3)C–CHPh](9),[2-Ph 2 P(4-Me-C 6 H 3)S] Ni [Ph(PMe 3)C–CHSiMeMe 3 ](10)或[2-Ph 2 P(4-Me-C 6 H 3)S] Ni [Me 3 Si(PMe 3)C–CHPh](10),[2-Ph 2 P(6-Me 3 Si-C 6 H 3)S] Ni [Ph(PMe 3)C– CHSiMe 3 ](11)或[2-Ph 2 P(6-Me 3 Si-C 6 H 3)S] Ni [Me 3 Si(PMe 3)C–CHPh](11)和[2-Ph 2 P(4-Me 3 Si-C 6 H 3)S] Ni [Ph(PMe 3)C–CHSiMe 3 ](12)或[2-Ph通过苯乙炔,二苯乙炔和1-苯基的反应形成了含碘配体的2 P(4-Me 3 Si-C 6 H 3)S] Ni [Me 3 Si(PMe 3)C–CHPh](12) -2-(三甲基甲硅烷基)乙炔与1,2,和3,分别。苯基/乙烯基交换镍(II)配合物[2-(PH(CH 2 ═CSiMe3)P(4-ME-C 6 H ^ 3)S]的Ni(PH)(PME 3)(13),[2-(PH(CH 2 ═CSiMe 3)P((6-ME 3的Si-C 6 ħ 3)] S的Ni(PH)(PME 3)(14),和[2-(PH(CH 2 ═CSiMe 3)P((4-ME 3的Si-C 6 H ^ 3)S]的Ni(PH) (PME 3)(15)可通过三甲基硅烷基的插入来获得成的Ni-H键1,2,和3。据我们所知,这是炔烃与氢化镍之间的一种新型反应类型。结果表明,增加苯环或炔烃上的电负性会导致乙烯基镍配合物的不稳定性,并且有利于通过乙烯基/苯基交换反应进行C-P还原消除形成镍环杂环丙烷配合物或苯基镍配合物。如果镍中心的电负性更高。所有的镍配合物,用IR,NMR和复合的分子结构完全检测到1,2,7,9,13,和14是由单晶X射线衍射证实。











































 京公网安备 11010802027423号
京公网安备 11010802027423号