当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nitrogenase Cofactor Assembly: An Elemental Inventory
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2017-10-24 00:00:00 , DOI: 10.1021/acs.accounts.7b00417 Nathaniel S. Sickerman 1 , Markus W. Ribbe 1, 2 , Yilin Hu 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2017-10-24 00:00:00 , DOI: 10.1021/acs.accounts.7b00417 Nathaniel S. Sickerman 1 , Markus W. Ribbe 1, 2 , Yilin Hu 1
Affiliation
|
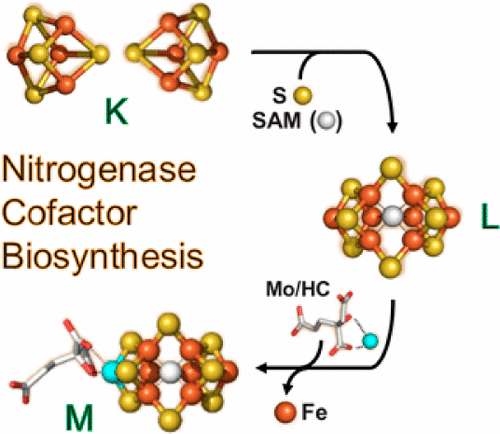
Nitrogenase is known for its remarkable ability to catalyze the reduction of N2 to NH3, and C1 substrates to short-chain hydrocarbon products, under ambient conditions. The best-studied Mo-nitrogenase utilizes a complex metallocofactor as the site of substrate binding and reduction. Designated the M-cluster, this [MoFe7S9C(R-homocitrate)] cluster can be viewed as [MoFe3S3] and [Fe4S3] subclusters bridged by three μ2-sulfides and one μ6-interstitial carbide, with its Mo end further coordinated by an R-homocitrate moiety. The unique cofactor has attracted considerable attention ever since its discovery; however, the complexity of its structure has hindered mechanistic understanding and chemical synthesis of this cofactor. Motivated by the pressing questions related to the structure and function of the nitrogenase cofactor, one major thrust of our research has been to unravel the key biosynthetic steps of this metallocluster to cultivate a deeper understanding of these reactions and their effects on functionalizing the cofactor.
中文翻译:

氮酶辅因子组装:基本清单
氮酶以其在环境条件下催化N 2还原为NH 3和C 1底物为短链烃产物的出色能力而闻名。研究最好的Mo-氮化酶利用复杂的金属因子作为底物结合和还原的位点。指定的M-簇,此[钼铁7小号9 C(ř -homocitrate)]簇可以被看作是[钼铁3小号3 ]和[铁4小号3 ]子群集桥接由三个μ 2 -sulfides和一个μ 6 -间隙碳化物,其Mo端进一步由R配位-均一的部分。自从发现以来,这种独特的辅因子就引起了广泛的关注。然而,其结构的复杂性阻碍了该辅因子的机械理解和化学合成。受与固氮酶辅因子结构和功能有关的紧迫问题的推动,我们研究的主要方向是阐明该金属簇簇的关键生物合成步骤,以加深对这些反应及其对辅因子功能化的影响的了解。
更新日期:2017-10-24
中文翻译:

氮酶辅因子组装:基本清单
氮酶以其在环境条件下催化N 2还原为NH 3和C 1底物为短链烃产物的出色能力而闻名。研究最好的Mo-氮化酶利用复杂的金属因子作为底物结合和还原的位点。指定的M-簇,此[钼铁7小号9 C(ř -homocitrate)]簇可以被看作是[钼铁3小号3 ]和[铁4小号3 ]子群集桥接由三个μ 2 -sulfides和一个μ 6 -间隙碳化物,其Mo端进一步由R配位-均一的部分。自从发现以来,这种独特的辅因子就引起了广泛的关注。然而,其结构的复杂性阻碍了该辅因子的机械理解和化学合成。受与固氮酶辅因子结构和功能有关的紧迫问题的推动,我们研究的主要方向是阐明该金属簇簇的关键生物合成步骤,以加深对这些反应及其对辅因子功能化的影响的了解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号