Talanta ( IF 5.6 ) Pub Date : 2017-10-23 , DOI: 10.1016/j.talanta.2017.10.032 G. Coussot , C. Faye , A. Le Postollec , M. Dobrijevic
|
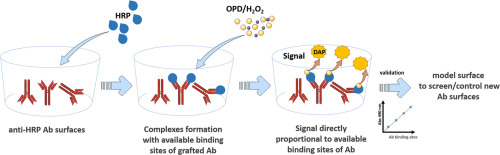
Antibody-coated surfaces (Ab surfaces) play a key role in bioanalytical tools developed for biosensors and diagnostics. Therefore, a high and well-defined functional activity of the Ab surface is required. The functional activity of the Ab surface depends on its available binding sites i.e. “the active sites” that are able to capture antigen (Ag). The number of active binding sites strongly depends on the immobilization strategy used to fix the Ab on the solid surface. Determination of layer thickness or surface topology are often used to characterize the Ab surfaces but there is no gold standard method for the study of the functionality of the Ab surfaces.
For that purpose, we aim at developing an assay allowing to determine the performances of Ab surfaces. In the present study, anti-horseradish peroxidase antibody (anti-HRP Ab) is used as capture Ab covalently bound to the surface and enzyme HRP as Ag. This direct assay permits, in one-step, to generate a signal utilizing the catalytic properties of HRP. The signal is directly proportional to the amount of HRP bound on the Ab surface, and therefore to the active binding sites of immobilized Ab. The HRP/anti-HRP Ab interactions may be a useful indicator to construct accurate and reproducible active Ab surfaces and also to improve their performances in term of stability and sensitivity.
Optimization of the assay parameters and quality of the results are presented. A good repeatability and an acceptable inter-day precision (RSD < 10%) are reported.
中文翻译:

以辣根过氧化物酶为抗原的一步式直接免疫分析法,用于研究抗体表面的功能
抗体包被的表面(Ab表面)在为生物传感器和诊断开发的生物分析工具中起着关键作用。因此,需要Ab表面的高度且功能明确的功能活性。Ab表面的功能活性取决于其可用的结合位点,即能够捕获抗原(Ag)的“活性位点”。活性结合位点的数量在很大程度上取决于用于将Ab固定在固体表面上的固定策略。层厚度或表面拓扑的确定通常用于表征Ab表面,但尚无用于研究Ab表面功能的金标准方法。
为此,我们旨在开发一种测定抗体表面性能的方法。在本研究中,抗辣根过氧化物酶抗体(抗HRP Ab)用作与表面共价结合的捕获Ab,酶HRP作为Ag。这种直接测定可以一步一步利用HRP的催化特性产生信号。信号与抗体表面上结合的HRP的量成正比,因此与固定抗体的活性结合位点成正比。HRP /抗HRP Ab相互作用可能是构建准确且可重现的活性Ab表面并改善其稳定性和敏感性方面的有用指标。
介绍了测定参数的优化和结果的质量。据报道具有良好的重复性和可接受的日间精度(RSD <10%)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号