Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2017-10-23 , DOI: 10.1016/j.bmcl.2017.10.055 Anna A. Hovhannisyan , The Hien Pham , Dominique Bouvier , Xiao Tan , SiAmmar Touhar , Gevorg G. Mkryan , Ashot M. Dallakyan , Chahrazade El Amri , Gagik S. Melikyan , Michèle Reboud-Ravaux , Michelle Bouvier-Durand
|
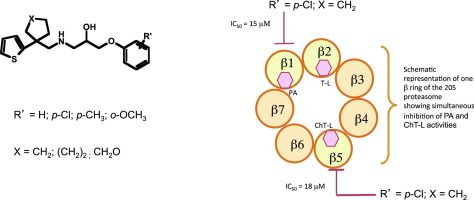
New series of thiophene-containing phenoxypropanolamines were synthesized and evaluated for their potency to inhibit the three proteolytic activities of the mammalian 20S proteasome. Noticeable inhibition of both ChT-L and PA activities was obtained with three compounds: one with unsubstituted phenoxypropanolamine group (7) and the two others with a p-Cl-substituted group (4 and 9). For three other compounds (3, 8 and 10), ChT-L activity alone was significantly inhibited. In silico docking performed on the β5 and β1 subunits bearing the respective ChT-L and PA catalytic sites showed features common to poses associated with active compounds. These features may constitute a selectivity criterion for structure-guided inhibitor design.
中文翻译:

苯氧基丙醇胺衍生物作为20S蛋白酶体β1和β5亚基的选择性抑制剂
合成了一系列新的含噻吩的苯氧基丙醇胺,并评估了它们抑制哺乳动物20S蛋白酶体的三种蛋白水解活性的能力。ChT-L和PA活性均受到三种化合物的显着抑制:一种具有未取代的苯氧基丙醇胺基团(7),另外两种具有对-Cl-取代基团(4和9)。对于其它三种化合物(3,8和10),CHT-L活性单独显著抑制。在计算机上对带有各自ChT-L和PA催化位点的β5和β1亚基进行的对接显示出与活性化合物相关的姿势共有的特征。这些特征可以构成用于结构指导的抑制剂设计的选择性标准。











































 京公网安备 11010802027423号
京公网安备 11010802027423号