当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Match-Making Reactors to Chemistry: A Continuous Manufacturing-Enabled Sequence to a Key Benzoxazole Pharmaceutical Intermediate
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2017-10-23 00:00:00 , DOI: 10.1021/acs.oprd.7b00254 Flavien Susanne 1 , Benjamin Martin 1 , Michel Aubry 1 , Joerg Sedelmeier 1 , Fabio Lima 1 , Serbuelent Sevinc 1 , Lorenzo Piccioni 1 , Julien Haber 1 , Berthold Schenkel 1 , Francesco Venturoni 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2017-10-23 00:00:00 , DOI: 10.1021/acs.oprd.7b00254 Flavien Susanne 1 , Benjamin Martin 1 , Michel Aubry 1 , Joerg Sedelmeier 1 , Fabio Lima 1 , Serbuelent Sevinc 1 , Lorenzo Piccioni 1 , Julien Haber 1 , Berthold Schenkel 1 , Francesco Venturoni 1
Affiliation
|
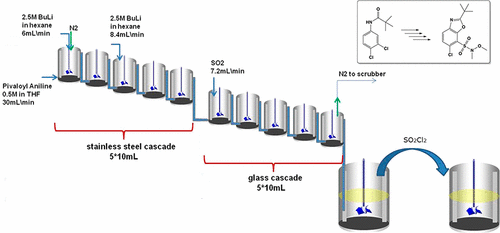
The focus of this study was to develop a chemical reaction sequence toward a key benzoxazole building block, required for clinical manufacturing of a lead candidate in the respiratory disease area. The chemistry consisted of initial low-temperature reactions with an organometallic reagent to generate the benzoxazole core, and was followed by noncryogenic transformations toward a sulfonamide substituent. With particular interest in continuous-flow manufacturing we attempted to integrate the entire sequence on lab scale. Subsequent in-depth process research, supported by PAT and calorimetry studies, revealed the critical parameters of each step, leading to a more rational attribution of mode of operation: flow, batch, or semibatch. Two bench-scale cascades of continuously stirred tank reactors (CSTRs) were constructed to meet the challenge of high exothermicity and solids formation and were key to smoothly upscaling the chemistry to deliver 17 kg of benzoxazole in superior yield, quality, and robustness.
中文翻译:

化学匹配的反应器:关键的苯并恶唑医药中间体的连续制造流程
这项研究的重点是开发针对关键苯并恶唑结构单元的化学反应序列,这是临床生产呼吸系统疾病领域的主要候选药物所必需的。化学过程包括与有机金属试剂的初始低温反应以生成苯并恶唑核,然后进行向磺酰胺取代基的非低温转化。对连续流制造特别感兴趣,我们尝试在实验室规模上整合整个序列。随后在PAT和量热法研究的支持下进行了深入的过程研究,揭示了每个步骤的关键参数,从而导致了操作模式(流量,间歇或半间歇)的更合理归因。
更新日期:2017-10-23
中文翻译:

化学匹配的反应器:关键的苯并恶唑医药中间体的连续制造流程
这项研究的重点是开发针对关键苯并恶唑结构单元的化学反应序列,这是临床生产呼吸系统疾病领域的主要候选药物所必需的。化学过程包括与有机金属试剂的初始低温反应以生成苯并恶唑核,然后进行向磺酰胺取代基的非低温转化。对连续流制造特别感兴趣,我们尝试在实验室规模上整合整个序列。随后在PAT和量热法研究的支持下进行了深入的过程研究,揭示了每个步骤的关键参数,从而导致了操作模式(流量,间歇或半间歇)的更合理归因。











































 京公网安备 11010802027423号
京公网安备 11010802027423号