Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2017-10-21 , DOI: 10.1016/j.bmcl.2017.10.050 Martin Krátký , Szilvia Bősze , Zsuzsa Baranyai , Jiřina Stolaříková , Jarmila Vinšová
|
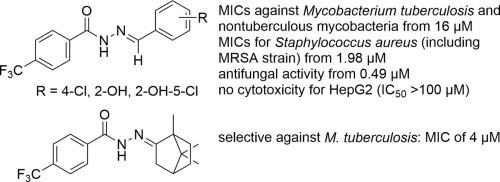
Reflecting the known biological activity of isoniazid-based hydrazones, seventeen hydrazones of 4-(trifluoromethyl)benzohydrazide as their bioisosters were synthesized from various benzaldehydes and aliphatic ketones. The compounds were screened for their in vitro activity against Mycobacterium tuberculosis, nontuberculous mycobacteria (M. avium, M. kansasii), bacterial and fungal strains. The most antimicrobial potent derivatives were also investigated for their cytostatic and cytotoxic properties against three cell lines. Camphor-based molecule, 4-(trifluoromethyl)-N′-(1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylidene)benzohydrazide, exhibited the highest and selective inhibition of M. tuberculosis with the minimum inhibitory concentration (MIC) of 4 µM, while N′-(4-chlorobenzylidene)-4-(trifluoromethyl)benzohydrazide was found to be superior against M. kansasii (MIC = 16 µM). N′-(5-Chloro-2-hydroxybenzylidene)-4-(trifluoromethyl)benzohydrazide showed the lowest MIC values for gram-positive bacteria including methicillin-resistant Staphylococcus aureus as well as against two fungal strains of Candida glabrata and Trichophyton mentagrophytes within the range of ≤0.49–3.9 µM. The convenient substitution of benzylidene moiety at the position 4 or the presence of 5-chloro-2-hydroxybenzylidene scaffold concomitantly with a sufficient lipophilicity are essential for the noticeable antimicrobial activity. This 5-chlorosalicylidene derivative avoided any cytotoxicity on two mammalian cell cultures (HepG2, BMMΦ) up to the concentration of 100 µM, but it affected the growth of MonoMac6 cells.
中文翻译:

4-(三氟甲基)苯并肼衍生物derived的合成及生物演化
为了反映基于异烟肼的的已知生物活性,由各种苯甲醛和脂肪族酮合成了17种4-(三氟甲基)苯并肼的bio酮作为其生物等排体。筛选化合物对结核分枝杆菌,非结核分枝杆菌(鸟分枝杆菌,堪萨斯分枝杆菌),细菌和真菌菌株的体外活性。还研究了最有效的抗微生物衍生物对三种细胞系的抑制细胞和细胞毒性的特性。基于樟脑的分子4-(三氟甲基)-N '-(1,7,7-三甲基双环[2.2.1]庚-2-亚基)苯并肼具有最高的选择性抑制作用结核分枝杆菌的最低抑菌浓度(MIC)为4 µM,而N '-(4-氯亚苄基)-4-(三氟甲基)苯甲酰肼则优于堪萨斯分枝杆菌(MIC = 16 µM)。N '-(5-氯-2-羟基苄叉)-4-(三氟甲基)苯并肼显示出革兰氏阳性细菌的最低MIC值,包括耐甲氧西林的金黄色葡萄球菌以及两种念珠菌和毛癣菌的真菌菌株在≤0.49–3.9 µM的范围内。对于第4位的亚苄基部分的方便取代或5-氯-2-羟基亚苄基支架的存在,同时具有足够的亲脂性,对于显着的抗微生物活性是必不可少的。此5- chlorosalicylidene衍生物避免在两个哺乳动物细胞培养物(HepG2细胞,BMM任何细胞毒性Φ)高达100μM的浓度,但它影响MONOMAC6细胞的生长。











































 京公网安备 11010802027423号
京公网安备 11010802027423号